

Safety Watch
Accountability through honest and investigative reporting.
Home News Devices Smith & Nephew’s Knee Rep...
Smith & Nephew’s Knee Replacement Device Recalled
Written by Emre Ertugrul
Smith & Nephew’s first-generation knee replacement device was recalled on Oct. 1 as the Food and Drug Administration announced the Class 2 device recall online, following a series of research and tests regarding the high failure rate of the device.
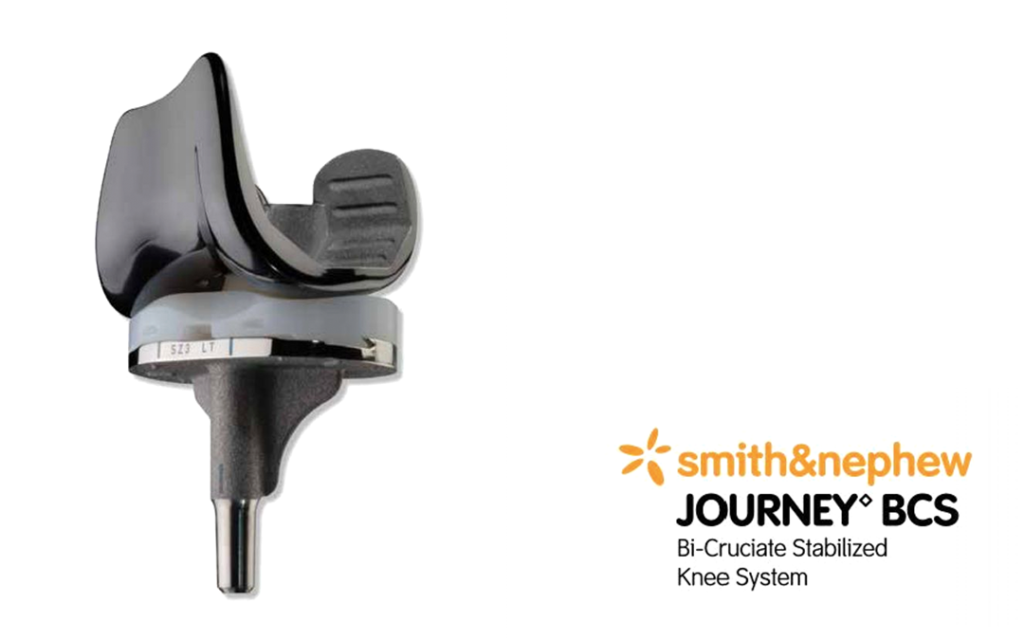
Smith & Nephew’s JOURNEY Bi-Cruciate Stabilized (BCS) Knee system was the first-generation version of the JOURNEY II BCS which replaced the former on global market.
The Field Safety Notice (FSN) sent to risk managers on June 13, requested customers to inspect and locate the affected devices with the purpose of their returns to Smith & Nephew, the FDA stated in the report. The same notification was also sent to surgeons.
Earlier in June, Smith & Nephew issued a safety notice regarding the high rate of failure of its first-generation device, due to early femoral and tibial insert component complications.
Total knee replacement is the process in which part of the knee joint is replaced with artificial knee parts – a common process that is experienced by hundreds of thousands of Americans each year.
“Review of post-market surveillance data” resulted in the urgent safety notice as the device reportedly caused unexpectedly earlier needs in patients for painful revision surgeries. The manufacturer stated that JOURNEY BCS device had the same reasons for revision as other knee systems on the market, however, the first-generation device had “a rate higher than expected.”
The first-generation system was released for market globally in 2013 and is no longer for sale.
In August, the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK issued a warning for providers and physicians that Smith & Nephew’s first-generation device has a 50% greater risk to cause early failure.
A clinical follow-up process conducted by Smith & Nephew concluded that the JOURNEY BCS Knee system has a revision rate over 1.5 times the average revision rates in the National Joint Registry of the UK and Australian Orthopaedic Association National Joint Replacement Registry.
The Journey II BCS Knee system will not be affected by the action.

Emre Ertugrul
Emre Ertugrul is a reporter for Safetywatch.org, covering controversial drugs and medical devices, reporting on health policy and the FDA. He studied journalism with concentration in investigative reporting at Boston University. Previous experience with the New England Center for Investigative Reporting include tax issues, racial profiling and criminal justice. He also worked as an international news intern at Milliyet Newspaper and is currently one of the editors for Gazet.com.
Get a Free Case Evaluation Now!
Your Name (required)
Your Email (required)
Your Phone (required)
Zip Code (required)
Your Message
Comments are closed.
Popular Searches
- Mesothelioma
- Toxic Water
- Defective Medical Devices
- Perry Weitz
- Arthur Luxenberg
Smith & Nephew Journey BCS Knee Replacement
Your first generation Smith & Nephew Journey Bi-cruciate Stabilized (BCS) Knee replacement system (Journey I BCS Knee System) may be defective. The manufacturer issued a voluntary recall in June of 2018, but phased this model out of production five years ago. If you had a first generation Smith & Nephew Journey BCS knee implanted, and the product failed, you may be entitled to compensation.
We are no longer accepting new cases.
How Weitz & Luxenberg Can Help
Weitz & Luxenberg is currently accepting clients who received a first generation Smith & Nephew Journey BCS knee and subsequently needed to undergo a revision, or replacement, surgery or have been advised by their doctor they need to undergo a revision surgery due to femoral component loosening. We invite you to contact us for more information about your legal options.
If you have undergone a knee replacement surgery, are having problems, and are not sure whether you received a first generation Smith & Nephew Journey BCS knee, we encourage you to ask your doctor which knee replacement model you received. Depending on your specific implant, you may be able to seek compensation.
First Generation Journey BCS Knee Complications
Revision surgeries are costly. You may also need to undergo follow-up treatment and physical therapy.
You may not be able to earn a living if your knee complications keep you disabled at home. Your total medical costs could become exorbitant.
Possible first generation Smith & Nephew Journey BCS knee implant complications for which you could receive compensation may include:
- Recommendation from your physician to undergo a revision surgery due to femoral component loosening.
- Revision surgery due to femoral component loosening.
Knee Implant Recall
Smith & Nephew voluntarily recalled its first generation Journey BCS Knee System on June 13, 2018. The U.S. Food and Drug Administration later categorized this recall as Class 2. (1) (2)
The manufacturer indicated in its Urgent Field Safety Notice that this knee was failing as a higher rate than other primary total knee arthroplasty implants. It noted that data in two joint registries “indicate that the first generation JOURNEY BCS Knee System has a revision rate over 1.5 times the primary total knee arthroplasty device class average revision rates reported in those registries.” (3) (4) (5)
In its Urgent Field Safety Notice Smith & Nephew asked customers to inspect inventory and locate and quarantine affected, unused devices. (6) (7) (8)
Field Safety Notice
In its Urgent Field Safety Notice, Smith & Nephew said the affected product was its first-generation Journey BCS, which was introduced to the market in 2005. The company phased this model out of production between 2013 and 2014. (9) (10)
Smith & Nephew said its “analysis of available post-market surveillance data suggests that patients that have been implanted with a first generation JOURNEY BCS Knee System may have a higher risk of requiring a revision earlier than they or their surgeon had expected.” (11) (12)
Design Failure
On July 23, 2018, the United Kingdom issued a medical device alert instructing against implanting Smith & Nephew’s first generation Journey BCS knee device and cautioned physicians to monitor patients regularly for up to 10 years for signs of device loosening. (13) (14)
In addition, the U.K.’s medical device alert notes the National Joint Registry of England, Wales, and Northern Ireland showed the revision rate for this knee device was more than double the average rate for primary total knee replacements. (15) (16)

What Studies Show
According to authors of one study partially funded by Smith & Nephew, Smith & Nephew’s first generation Journey BCS had a significantly higher rate of revision than Smith & Nephew’s Journey II knee. The Journey I had a risk of revision over four times higher than the Journey II. (17) (18)
Hear From Our Clients
I chose Weitz & Luxenberg because the firm has a reputation for working really hard. I went with them because I didn’t want just anybody representing me. I felt that, if I was going to sue, I wanted a firm that had a lot of experience and a lot of resources. I wanted the big guns.”
Implant Litigation
Weitz & Luxenberg has been litigating cases involving defective medical devices for decades. As a national firm, we are prepared to handle large-scale medical device litigation across the country.
Our attorneys make it their mission to help clients harmed by defective medical products any medical device corporations create and promote. They are specially trained to handle complex medical device lawsuits.
If you were implanted with a defective knee replacement, we may be able to seek appropriate compensation on your behalf. Over the years, we have secured millions of dollars on behalf of clients injured by faulty medical devices.
For more information, we encourage you to contact us at (833) 977-3437 .
Request a Free Legal Consultation
You may be entitled to legal compensation. We would be honored to represent you.
Request A Free Consultation
" * " indicates required fields
* While our past record doesn't guarantee future success, it is something you may want to consider when evaluating our experience.
- U.S. Food & Drug Administration. (2018, October 1). Class 2 Device Recall. Journey BCS Knee System. Retrieved from https://www.accessdata.fda.gov/SCRIPTs/cdrh/cfdocs/cfres/res.cfm?id=165170
- U.S. Food & Drug Administration. (2018, October 1). Class 2 Device Recall. Journey BCS Knee System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=165169
- MHRA. (2018, June 13). Smith & Nephew. Urgent Field Safety Notice. Retrieved from https://www.moph.gov.lb/userfiles/files/Medical%20Devices/Medical%20Devices%20Recalls%202018/3-7-2018/JOURNEYIIBCSKneeSystem.pdf
- Smith & Nephew, Inc. (2018, June 13). Urgent Field Safety Notice. Retrieved from https://www.moph.gov.lb/userfiles/files/Medical%20Devices/Medical%20Devices%20Recalls%202018/3-7-2018/JOURNEYIIBCSKneeSystem.pdf
- Eisner, W. (2018, August 8). Safety Notice for S&N Journey BSC Knee. Retrieved from https://ryortho.com/breaking/safety-notice-for-sn-journey-bsc-knee/
- GOV.UK. (2018, July 23). First generation JOURNEY BCS Knee System—Higher than expected risk of revision (MDA/2018/026). Retrieved from https://web.archive.org/web/20180816121155/https://www.gov.uk/drug-device-alerts/first-generation-journey-bcs-knee-system-higher-than-expected-risk-of-revision-mda-2018-026
- Christen, B., & Kopjar, B. (2018, August 25). Second-generation bi-cruciate stabilized total knee system has a lower reoperation and revision rate than its predecessor. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30167859

- Our Attorneys
- Giving Back
- Depo-Provera ®
- Bard ® PowerPort TM
- Hip Replacement
- Exactech Knee and Ankle
- Hernia Mesh
- Allergan Biocell Breast Implant
- Toxic Baby Food
- Enfamil ® and Similac ® Baby Formula
- Hair Straightener
- Social Media Harm
- AFFF Firefighting Foam
- Workers’ Compensation
- Nursing Home Abuse
- Assisted-Care Abuse
- Motor Vehicle Accidents
- Birth Injuries
- Medication Errors
- Misdiagnosis
- Surgical Errors
- Social Security Disability
Smith & Nephew Knee Lawsuit
Please visit our main knee lawsuits page to see which types of knee-related litigations we are currently accepting.
Did you receive a Smith & Nephew Journey™ I BCS Knee and are now facing, or have had, revision surgery? If so, and you are considering filing a Smith & Nephew Knee lawsuit, we may be able to help.
The first generation model of Smith & Nephew’s Bi-cruciate Stabilized (BCS) Knee replacement system—the Journey I BCS Knee System—has had a high failure rate due to femoral component loosening. If you were implanted with the Journey I BCS Knee and have had or been told you need revision surgery, you may be entitled to compensation.
Trustwell Law is accepting cases on behalf of patients who received a first generation Smith & Nephew Journey BCS Knee and have had or been told they need revision surgery. Contact us at 800-796-1636 or submit your case details online for a free consultation, and someone from our legal team will contact you shortly. You may be entitled to compensation.
Our attorneys have years of experience and a reputation for personalized, compassionate partnering with our clients. We also have access to the expertise, resources, and manpower to fully investigate your circumstances in order to get you the justice you deserve.
Smith & Nephew Journey I BCS Knee Replacements
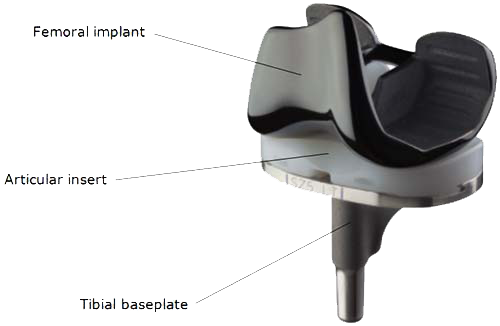
The first-generation Smith & Nephew BCS Knee, the Journey I BCS Knee System, was first sold in 2005 for partial and total knee replacements. Marketing materials indicated the line was aimed at younger, more active patients. But the UK-based company began phasing out the Journey I model in 2013 and 2014. Following the Journey I, Smith & Nephew introduced the Journey II.
Smith & Nephew Journey I BCS Knee Recall
In June 2018, Smith & Nephew voluntarily issued a recall for the Journey I BCS Knee. In the Urgent Field Safety Notice the company released, it explains that the knee was found to have a revision rate one and a half times higher than similar knee implants. The company further stated that recipients of the device could need revision surgery earlier than they or their doctors would normally expect. The Notice also cautioned medical practitioners and facilities that had purchased the unit to find and set aside any unused devices.
The U.S. Food and Drug Administration (FDA) later classified this recall as a Class II recall: “a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”
Smith & Nephew Journey I BCS Knee Replacement Issues and Warnings
In July 2018, the United Kingdom, where Smith & Nephew is based, issued a medical device alert concerning the Journey I BCS Knee replacement system. The alert instructed medical practitioners not to implant the Journey I BCS Knee, and it further advised physicians who had already implanted the device to monitor their patients carefully for up to ten years—to watch for signs of femoral component loosening and implant destabilization.
The alert related that the National Joint Registry of England, Wales, Northern Ireland, and the Isle of Man had found the revision rate of the Journey I BCS Knee to be more than double the average rate for primary total knee replacements. And yet another study, partially funded by Smith & Nephew, found the revision rate to be four times greater for the Journey I knee as that of Smith & Nephew’s next release—the Journey II.
Smith & Nephew Journey I BCS Revision Surgery and Litigation
Revision, or replacement, surgery is painful and costly. In addition, having endured the original surgery for the hope of improved quality of movement and life that lasts many years, it is particularly disheartening to have to face revision surgery long before it would normally be expected.
If you or a loved one received a Smith & Nephew Journey I BCS Knee and have had or been advised that you need revision surgery due to femoral component loosening, contact us . You may be entitled to compensation for pain and suffering, medical and other expenses and lost work.
- Christen, B., and B. Kopjar. (2018, August 25). Second-generation bi-cruciate stabilized total knee system has a lower reoperation and revision rate than its predecessor. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30167859
- Eisner, W. (2018, August 8). Safety Notice for S&N Journey BSC Knee. Retrieved from https://ryortho.com/breaking/safety-notice-for-sn-journey-bsc-knee/
- GOV.UK. (2018, July 23). First generation JOURNEY BCS Knee System—Higher than expected risk of revision (MDA/2018/026). Retrieved from https://www.gov.uk/drug-device-alerts/first-generation-journey-bcs-knee-system-higher-than-expected-risk-of-revision-mda-2018-026
- MHRA. (2018, June 13). Smith & Nephew. Urgent Field Safety Notice. Retrieved from https://mhra.filecamp.com/public/file/3l5k-3don01i7
- Smith & Nephew, Inc. (2018, June 13). Urgent Field Safety Notice. Retrieved from https://www.mcgartland.com/wp-content/uploads/2018/07/Smith-Nephew-Field-Safety-Notice-Journey-BCS-Knee.pdf
- U.S. Food & Drug Administration. (2018, October 1). Class 2 Device Recall. Journey BCS Knee System. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=165169
- U.S. Food & Drug Administration. (2014, July 31). Recalls Background and Definitions. Retrieved from https://www.fda.gov/safety/industry-guidance-recalls/recalls-background-and-definitions
Free Case Evaluation
You will never be charged a fee unless a recovery is made for you.

Smith & Nephew Journey Knee Recalls

Smith & Nephew (S&N) has announced several recalls for the Journey knee replacement . The problem with some implants is a defective tibial baseplate or femoral implant. If these components break, patients may develop severe pain, instability, and require revision surgery.
Journey BCS Knee Recall in Australia
January 6, 2014 — The Journey Bi-Cruciate Stabilized (BCS) knee replacement system has been recalled by the Australian Therapeutic Goods Administration (TGA).
After reviewing a national database of joint implants, health officials recalled the femoral implant / tibial baseplate combination of the Journey BCS due to an unacceptably high rate of failure and revision surgery:
- Revision rate of 1.59 per 100 observed component years, compared to 0.72 for other total knee replacements.
- Yearly cumulative revision rate of 7% at five years post-implantation, compared to 3.8% for all other total knee replacements.
- Higher incidence of revision surgery for patellofemoral pain, unspecified pain, and instability.
Journey II Uni Tibial Baseplate Recall
March 3, 2010 — The U.S. Food and Drug Administration (FDA) has recalled Journey II Uni Tibial Baseplates . The Class II recall affects approximately 40,000 Journey II knee implants. Smith & Nephew has received complaints about baseplates breaking. When this occurs, patients must undergo revision surgery. If they do not, they could develop instability and premature wear of the implant.

Study Links Journey Deuce to High Failure Rate
September 2011 — The Journey of Arthroplasty has published a study of 36 people implanted with the Smith & Nephew Journey Deuce knee implant. They found that 14% of patients needed revision surgery within two years, including one patient who had a “catastrophically failed tibial baseplate.” In addition, 31% of patients reported poor results, and 53% said they would not have the surgery again.
Symptoms of Knee Replacement Failure
- Pain, swelling of knee or ankle
- Change in alignment of knee
- Loosening or instability
- Osteolysis (bone loss)
- Bone fractures
- Decreased knee function
- Stiffness in knee
- Damage to nerves or blood vessels
- Deep Vein Thrombosis (DVT)
- Pulmonary Embolism (PE)
Related Posts
- Journey BCS Knee Lawsuit
- Smith & Nephew Journey BCS Knee Implant Recall in Australia
- Bard Eclipse IVC Filter Lawsuit
- Study Links Statins and 12% Increased Risk of Diabetes
- Xarelto Recall Issued for Microbial Contamination
Free Case Evaluation

No matter what type of case you have, you may contact us with confidence by filling out the email contact form below or calling us directly by dialing toll free 24 hrs/day (866) 920-0753 .
- First Name *
- Last Name *
- Case Description
Search the Site

Contact Us With Confidence

- Million Dollar Advocates Forum
- Super Lawyers
- Best Lawyers in America
- Lawdragon Top 500 Leading Lawyers in America
- "Presidents Club"
- American Association for Justice
- American Bar Association
- American Board of Trial Advocates
- American College of Trial Lawyers
- International Academy of Trial Lawyers
- International Society of Barristers

Device Recall Information
Medical device recalls.
MARCQI posts Class I and Class II recalls that involve implantable devices because of the risk to patients. For reference, the definitions of these classes, according to the Food and Drug Administration (FDA), are:
- Class 1 recall: a situation in which there is a reasonable probability that the use of or exposure to a volatile product will cause serious adverse health consequences or death.
- Class 2 recall: a situation in which use of or exposure to a volatile product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.
- Class 3 recall: a situation in which use of or exposure to a violative product is not likely to cause adverse health consequences.
- Medical Device Safety Alert: issued in situations where a medical device may present an unreasonable risk of substantial harm. In some case, these situations also are considered recalls.
- Correction or Removal: manufacturers and importers are required to make a report to FDA of any correction or removal of a medical device(s) if the correction or removal was initiated to reduce a risk to health posed by the device or to remedy a violation of the Act caused by the device which may present a risk to health.
2020 Class I Recalls (updated 3/17/2023)
September 2020
Recall # Z-2941-2020
- MicroPort Orthopedics Inc. states that there have been reports of fractures of the long and extra-long Titanium modular femoral neck component after implantation
2024 Class II Recalls (updated 10/7/2024)
October 2024
Recall # Z-3171-2024
- DIAMOND POINT, BOTH ENDS K-WIRE, REF 128042, metallic bone fixation fastener, Batch 23GNX0077 – Packaging error: a package contained K-WIRE .062X9 2PT DM wires instead of K-WIRE .045X9 2PT DM as described on the product label.
Recall # Z-3172-2024
- DIAMOND POINT, BOTH ENDS K-WIRE, REF 128062, metallic bone fixation fastener, Batch 21KNX0074 – Packaging error: a package contained K-WIRE .062X9 2PT DM wires instead of K-WIRE .045X9 2PT DM as described on the product label.
September 2024
Recall # Z-3132-2024
- Biolox Option Taper Sleeve, Type 1 Taper, Standard Neck, Item Number: 650-1066, Lot Number: 3185263 – Mislabeled: Incorrect sleeve in the packaging. The outer packaging is labeled as a standard neck sleeve, however, the product within the box is a -6mm neck sleeve, and vice versa. The neck of the sleeve can be identified by the device etching as either STD or -6.
Recall # Z-3133-2024
- Biolox Option Taper Sleeve, Type 1 Taper, -6mm Neck, Item Number: 650-1064, Lot Number: 3185266 – Mislabeled: Incorrect sleeve in the packaging. The outer packaging is labeled as a standard neck sleeve, however, the product within the box is a -6mm neck sleeve, and vice versa. The neck of the sleeve can be identified by the device etching as either STD or -6.
Recall # Z-2410-2024
- Endo Model M Tibial Components Modular Knee Prosthesis System, Model/Catalog Number: Various sizes, all product lots manufactured since 01-Jun-2022 – Process design, blind screws of the modular tibial component cannot be loosened intraoperatively, prolongation surgery due to intraoperatively change in procedure, probably to cementing technique.
Recall # Z-2411-2024
- Endo Model SL Tibial Components, Model/Catalog Number: Various sizes, all product lots manufactured since 01-Jun-2022 – Process design, blind screws of the modular tibial component cannot be loosened intraoperatively, prolongation surgery due to intraoperatively change in procedure, probably to cementing technique.
August 2024
Recall# Z-2450-2024
- CPT HIP Joint Metal – Polymer Semi-Constrained Cemented Prosthesis, used in Hip Arthroplasty – Affected product has an increased risk of postoperative perisprosthetic femoral fracture (PFF). The IFU is being updated to reflect the risk of PFF.
Recall # Z-1934-2024
- LOSPA Tibial Insert – Model/Catalog Number: Various sizes – Due to unsupported 10 year expiration date.
Recall # Z-1935-2024
- LOSPA Patella Componenet – Model/Catalog Number: 01.10.9XX – Due to unsupported 10 year expiration date
Recall # Z-1265-2024
- NO 4 TRIATHLON TS PLUS TIB INS X3 POLY 16 MM, Catalog number 5537-G-416-E, Potential packaging breaches of inner blister and outer sterile blister.
Recall # Z-1625-2024
- TRIDENTII HEMI CLUSTER54E, Catalog number 702-11-54E – The acetabular shell may have excessive deburring, resulting in a smooth surface on the edge of the shell
Recall # Z-1624-2024
- TRIDENTII HEMI CLUSTER52E, Catalog number 702-11-52E – The acetabular shell may have excessive deburring, resulting in a smooth surface on the edge of the shell.
Recall # Z-1622-2024
- TRIDENTII HEMI CLUSTER48D, Catalog number 702-11-48D – The acetabular shell may have excessive deburring, resulting in a smooth surface on the edge of the shell.
Recall # Z-1260-2024
- Triathlon Total Knee System, X3 TRIATHLON CS INSERT NO 6 10 MM – The reason for the recall is potential packaging breaches of the inner blister and outer sterile blister
Recall # Z-1298-2024
- SPII Model Lubinus, Long Stem Prosthesis Standard Neck, cemented – Due to two complaints, additional guidance is required on the correct interpretation of the carton label Size and Type columns to avoid misinterpretation during surgery.
Recall # Z-1297-2024
- SPII Model Lubinus, Hip Prosthesis Stem XL Neck, cemented – Due to two complaints, additional guidance is required on the correct interpretation of the carton label Size and Type columns to avoid misinterpretation during surgery.
Recall # Z-1296-2024
- SPII Model Lubinus, Hip Prosthesis Stem Standard Neck, cemented – Due to two complaints, additional guidance is required on the correct interpretation of the carton label Size and Type columns to avoid misinterpretation during surgery.
2023 Class II Recalls (updated 11/28/2023)
November 2023
Recall # Z-0214-2024
- Unity Total Knee System, Model Number 112.001.34.- Potential for Unity CR Inserts Right size 6 from batch 529803 to be incorrectly labeled as Unity CR Inserts Right size 7 from batch 532405 and visa versa.
Recall # Z-0213-2024
- Unity CR Femur Right, Size 6, Model Number 112.001.32 – Potential for Unity CR Inserts Right size 6 from batch 529803 to be incorrectly labeled as Unity CR Inserts Right size 7 from batch 532405 and visa versa
Recall # Z-0158-2024
- JOURNEY II BCS CONSTRAINED ARTICULAR INSERT Size 5-6, 10 MM Left, REF 74029262; knee prosthesis – The JRNY II BCS XLPE ART ISRT SZ 5-6 LT 10MM was laser etched, labeled and packaged as a JRNY II BCS CNSTRD ART ISRT 5-6 LT 10MM. Also, a JRNY II BCS CNSTRD ART ISRT 5-6 LT 10MM was laser etched, labeled, and packaged as a JRNY II BCS XLPE ART ISRT SZ 5-6 LT 10MM.
Recall # Z-0157-2024
- JOURNEY II BCS Articular Insert REF 74027262; knee prosthesis- The JRNY II BCS XLPE ART ISRT SZ 5-6 LT 10MM was laser etched, labeled, and packaged as a JRNY II BCS CNSTRD ART ISRT 5-6 LT 10MM. Also, a JRNY II BCS CNSTRD ART ISRT 5-6 LT 10MM was laser etched, labeled, and packaged as a JRNY II BCS XLPE ART ISRT SZ 5-6 LT 10MM
October 2023
Recall # Z-2519-2023
- Zimmer – M/L Taper Hip Prosthesis Extended Offset, Reduced Neck Length, Size 4 and Size 6 – Reason for recall is mixed-up of materials/components. The outer package labeling and product etch are for a Size 6, however, the implant is a Size 4, and vice versa.
Recall # Z-1673-2023
- ENGAGE Cementless Partial Knee System, Coated Tibial Insert- Recent complaint data indicates that the revision rate may be trending higher than corresponding similar devices in global joint replacement registries. The data identifies a potential signal that the performance is an outlier versus the state of the art with respect to the risk for revision.
Recall # Z-1672-2023
- ENGAGE Cementless Partial Knee System, Coated Tibial Tray, Part Numbers: a) REF 1-10012-100, SIZE 1-LEFT MEDIAL- Recent complaint data indicates that the revision rate may be trending higher than corresponding similar devices in global joint replacement registries. The data identifies a potential signal that the performance is an outlier versus the state of the art with respect to the risk for revision.
Recall # Z-1671-2023
- Smith & Nephew – ENGAGE Cementless Partial Knee System, Tibial Anchor Stem, Part Numbers: a) REF 1-10011-100 (SIZE 1-2), b) REF 1-10011-200 (SIZE 3-4), c) REF 1-10011-300 (SIZE 5-6); Unicondylar knee prosthesis- Recent complaint data indicates that the revision rate may be trending higher than corresponding similar devices in global joint replacement registries. The data identifies a potential signal that the performance is an outlier versus the state of the art with respect to the risk for revision.
Recall # Z-1670-2023
- Smith & Nephew – ENGAGE Cementless Partial Knee System, Porous Femoral components, various sizes- Recent complaint data indicates that the revision rate may be trending higher than corresponding similar devices in global joint replacement registries. The data identifies a potential signal that the performance is an outlier versus the state of the art with respect to the risk for revision.
Recall # Z-1393-2023
- JOURNEY II UNI XLPE TIBIA INSERT MEDIAL SZ 7-8 8MM A mispack occurred during the manufacturing process, resulting in the box incorrectly containing JOURNEY II UNI Tibia Insert Medical Size 1-2 12MM instead of the JOURNEY II UNI Tibia Insert Medial Size 7-8 8MM.
Recall # Z-1294-2023
- CoCr Femoral Head, XS, 38/-8, Taper 12/14-Intended to be used as a modular head component for articulation in total hip arthroplasty. Item Number: 01.01012.384 received an Update the compatibility matrix as referred to in the Instructions for Use (IFU) for the CoCr Femoral Head XS- removes the compatibility with the Epsilon Durasul Constrained Acetabular Liners from the matrix due to the range of motion in flexion/extension being less than 100 degrees as recommended per an internationally recognized standard
Recall # Z-1284-2023
- G7 Acetabular System, Dual Mobility Acetabular Liner, 40mm, Size D, Model # 110024462 has an outer package where the labeling and product etch are a 40mm Site D liner, however, the implant is a 38mm Size C liner
Recall # Z-1263-2023 , Z-1264-2023 , Z-1265-2023 , Z-1266-2023 , Z-1267-2023
- Attune Revision Tibial Insert (various sizes) received a higher than specified irradiation dose. This exceeds the validated range for exposure to gamma radiation of these devices and may result in changes to the implant material properties
Recall # Z-1733-2022 , Z-1732-2022 , Z-1731-2022 , Z-1730-2022 , Z-1734-2022 , Z-1728-2022 , Z-1727-2022 , Z-1726-2022, Z-1729-2022
- Specific GLX acetabular polyethylene liners, packaged in non-conforming bags, may adversely impact the device and contribute to accelerated wear
Recall # Z-1850-2021 , Z-1851-2021
- Corin TriFit has units from one batch where TS size 2 stem were found to be labeled as a TriFit CF size 7 stem. And vice versa.
Recall # Z-1509-2022
- Corin METAFIX size 7 collarless stem from batch 478179 which was incorrectly labelled as a MetaFix size 3 collared stem from batch 485630
Recall # Z-1725-2022 , Z-1726-2022 , Z-1727-2022
Recall # Z-0007-2023
- G7 Acetabular System, Acetabular Shell has outer sterile package cavity with a corner wall thickness that is below the specification. this corner wall could potentially crack during transit. potential risks include non-clinically or clinically significant extension of surgery or infection leading to surgical intervention
Recall # Z-0275-2023 , Z-0276-2023
- Evolution MP Tibial Bases, various sizes has 1 confirmed incident where ETPKN2PL lot 1916559, size 2 Evolution MP Tibial Base, was opened during surgers and contained ETPKN7SL lot 1916715, size 7 Evolution MP Tibial Base, in the packaging
Recall # Z-0273-2023
- The Utility Total Knee System’s internal packaging system for devices may have damage to the blisters potentially damaging the device or compromising the sterility of the packaging
Recall # Z-0466-2023 , Z-0467-2023 , Z-0468-2023 , Z-0469-2023 , Z-0470-2023 , Z-0471-2023
- Nextgen option stemmed tibial component, various sizes, has clinically and statistically significant higher overall revision rates when these tibial components are used with either the legacy posterior stabilized flex or LPS flex gender solutions femoral components as compared to other total knee arthroplasties in the United Kingdom National joint registry
Recall # Z-0951-2023
- Biostop F Bioresorbable Cement Restrictor is being removed as a precautionary measure because tested endotoxin levels were higher than recommended by the current FDA regulatory guidance…endotoxins have a potental to initiate inflammatory responses, ranging from a mild fever to potentially impact or damage to vital organs
Recall # Z-0726-2023 , Z-0727-2023
- Corin BIOLOX Delta Mod Head size 36xl is labelled as the size 32xl and vice-versa
2022 Class II Recalls (updated 3/18/2022)
Recall # Z-0670-2022
- Acos Modular Revision Hip System, Standard Cone Prox Body, Porous Plasma in scope underwent an incorrect rework operation for the porous plasma sprayed coating. Internal testing indicated that the parts may not have sufficient adhesion strength
2021 Class II Recalls
November 2021
Recall # Z-0160-2022 , Z-0159-2022
- Limacorporate S.P.A. Bone Screw/ Vite, various sizes, has a potential that the length of bone screws identified on labeling may not correspond to the actual length of the screw included
September 2021
Recall # Z-2515-2021 , Z-2514-2021 , Z-2509-2021 , Z-2510-2021 , Z-2511-2021 , Z-2512-2021 , Z-2513-2021
- Univation X System knee implant devices could malfunction, loosening the implant resulting in a potential revision surgery
Recall # Z-2348-2021
- Package labeled as 16mm x 80mm contained a 16mm x 130mm stem instead and result in obtaining a replacement
August 2021
Recall #s Z-2264-2021 , Z-2275-2021 , Z-2265-2021 , Z-2264-2021 , Z-2263-2021 , Z-2262-2021 , Z-2266-2021 , Z-2271-2021 , Z-2272-2021 , Z-2270-2021
- Arcos Proximal Cone Bodies could potentially exhibit chatter in the inner taper fretting corrosion and device failure in vivo leading to surgical intervention
Recall #s Z-2273-2021 , Z-2269-2021 , Z-2268-2021 , Z-2267-2021 , Z-2261-2021 , Z-2262-2021 , Z-2260-2021 , Z-2259-2021 , Z-2274-2021
- o Arcos Proximal Cone Bodies could potentially exhibit chatter in the inner taper fretting corrosion and device failure in vivo leading to surgical intervention
Recall #s Z-2250-2021 , and Z-2251-2021
- o Biolox Delta Femoral Head was packaged and labeled as 170-36-00 (36mm +0), but the device was marked as 10-36-03 (36mm +3.5)
Recall #s Z-2242-2021
- The nail head may become detached during surgery.
Recall #s Z-2133-2021 , Z-2132-2021 , Z-2115-2021 , Z-2116-2021 , Z-2117-2021 , Z-2118-2021 , Z-2119-2021 , Z-2120-2021 , Z-2121-2021 , Z-2122-2021 , Z-2123-2021 , Z-2124-2021 , Z-2126-2021 , Z-2127-2021 , Z-2128-2021 , Z-2129-2021
- Risk of edge-loading and premature prosthesis wear is possible in a specific subset of patients with certain implant configurations and surgical implant positioning
Recall # Z-2107-2021
- Not properly sterilized, because the outer pouch seal on the Tyvek header was sealed closely to the foil pouch
Recall # Z-2774-2020
- Due to an inconsistency in the raw material process, specific lots may contain units with internal non-homogenous material defects.
Recall # Z-0118-2021
- The anterior locking detail does not meet its design specifications.
Recall #s Z-2331-2020 , Z-2332-2020 , Z-2333-2020 , Z-2334-2020 , Z-2335-2020 , Z-2336-2020
- A manufacturing error resulted in out of specification R3 Acetabular Shells
February 2021
Recall # Z-0802-2021
- Certain Pinnacle Cup devices may potentially exhibit an oversized “minor diameter”, which could lead the Apex HE to thread through the shell of the cup without stopping or to protrude internally as a result of “cross-threading”. If cross-threading occurs, physicians will not feel the positive stop of the Apex HE against the cup.
2020 Class II Recalls
November 2020
Recall # Z-0332-2021
- Sterilant (hydrogen peroxide) used in acetabular cup hip prosthesis component was not evaluated to confirm the biocompatibility of the residual sterilant. Cannot rule out the potential for adverse tissue reactions
Recall # Z-0344-2021
October 2020
Recall # Z-0057-2021
- The outer package is labeled as a Size C 38 mm, however, the implant inside the package is a Size E 42 mm
Recall # Z-0081-2021
- Product not properly being aligned with the adequate gamma sterilization group and result in the product not being properly sterilized
Recall # Z-0097-2021
- RingLoc Bi-Polar Hip System Acetabular Cup ArCom: Acetabular Bi-Polar Cup, E1 Antioxidant Infused, 28 MM, 41 MM: Item No. 110010458; Lot No. 710930; UDI No. (01) 00880304568747 (17) 230614 (10) 710930
- RingLoc Hip system Acetabular Bi-Polar Cup: Acetabular Cup, ArCom, 41 MM OD, 28 MM ID: Item No. 11-165206, Lot No. 649750; UDI No. (01) 00880304001923 (17) 230614 (10) 649750
- The affected lots were gamma sterilized twice. Sufficient data does not exist to support the functionality, shelf life, or package integrity for more than one-time sterilization.
August 2020
Recall # Z-2948-2020
- MicroPort Orthopedics Inc. is voluntarily recalling any existing inventory of PROFEMUR¿ Titanium and Cobalt Chrome modular necks with previous package insert versions to replace the PROFEMUR¿ Hip System Package Insert with the most recent revision (150803-8)
Recall # Z-2849-2020
- Outer box labels for kit 0469247 correct, however the boxes contained the implants and jigs for 0468920
Recall # Z-2145-2020
- Potential presence of elevated endotoxin levels that exceed the specification limit
Recall # Z-2146-2020
Recall # Z-2147-2020
Recall # Z-2149-2020
Recall # Z-2150-2020
- 183622 Vanguard Knee System PS Tibial Bearing, 12 MM X 63/67 MM
- 183620 Vanguard Knee System PS Tibial Bearing, 10 MM X 63/67 MM
- 189048 Vanguard Knee System, AS Tibial Bearing, 18 MM X 67 MM
Recall # Z-2151-2020
- 189260 Vanguard Knee System, CR-L Mono Lock Tibial Bearing, 10 MM X 71 MM
- 189720 Vanguard Knee System, CR Mono Lock Tibial Bearing, 10 MM X 83 MM
- 189320 Vanguard Knee System, CR-L Mono Lock Tibial Bearing, 10 MM X 83 MM
- 189700 Vanguard Knee System, CR Mono Lock Tibial Bearing, 10 MM X 79 MM
Recall # Z-2152-2020
Recall # Z-2153-2020
Recall # Z-2154-2020
- Zimmer Biomet- Knee Products:
1) 184762 Knees Vanguard Knee System, Series-A Standard Patella, 28 MM; 2) 184764 Knees Vanguard Knee System, Series-A Standard Patella, 31 MM
Recall # Z-2155-2020
Recall # Z-2156-2020
Recall # Z-1478-2020
- Low Density Polyethylene (LDPE) particles may be present on the surface of the implant upon the opening of the inner packaging
Recall # Z-1479-2020
Recall # Z-1480-2020
Recall #: Z-1481-2020
- Low Density Polyethylene (LDPE) particles may be present on the surface of the implant upon the opening of the inner packaging.
Recall # Z-1482-2020
2019 Class II Recalls
- Two lengths of the 6.5mm RingLoc Hip System Self-Tapping Bone Screw may have the potential for the 20mm screws to be packaged as 30mm screws and vice versa.
- Two lengths of the 6.5mm RingLoc Hip System Self-Tapping Bone Screw may have the potential for the 30mm screws to be packaged as 20mm screws and vice versa.
2018 Correction or Removal
- Zimmer M/L Taper Hip Prosthesis, 7711 Series, Fem Stem 12/14 Neck Taper, Sz 4
- Zimmer M/L Taper Hip Prosthesis, 7711 Series, Fem Stem 12/14 Neck Taper, Sz 7.5
2018 Class 2 Recalls
- The label master file was errantly set up as a 63/37mm instead of a 63/67mm. There is no 63/37 size offered, and the product is laser marked with the correct size.
- Two lots of tibial bearings were commingled. There is a possibility that a 14mm tibial bearing is packaged in a box labeled as 12 mm tibial bearing and vice versa.
- One lot of 75mm tibial tray is potentially etched and labeled as a 79 mm tibial tray.
- Error in labeling. The labeling of the implant box outer label states the correct size of the implant. The patient sticker located inside the outer box states the incorrect size of the implant.
- There is a potential for debris in the hole on the superior lateral aspect of the device as a result of machining which may not have been adequately removed during the subsequent cleaning process.
- Zimmer Ringloc CoCr Modular Femoral Heads -6mm offset
- Zimmer Vanguard CR Porous Femoral 32.5mm LT Femur
- Depuy SIGMA HP PFJ Cemented Trochlear Implant
- Zimmer Biomet VERSYS BEADED FULLCOAT CALCAR HIP PROSTHESIS
- Zimmer Biomet VERSYS 10 INCH BEADED FC REV 15.0X250MM BOWED LT & RT
- Zimmer Biomet VERSYS 10 INCH BEADED FC REV 13.5X250MM BOWED LT and RT
- Zimmer Biomet VERSYS 7.5 INCH BEADED FC REV 13.5X190MM STRAIGHT
- Zimmer Biomet VERSYS 6 INCH BEADED FC 15X160MM STD BODY STD NECK
- Zimmer Biomet VERSYS 6 INCH BEADED FC 14X160MM STD BODY EXT & STD NECK
- Zimmer Biomet VERSYS 6 INCH BEADED FC 13X160MM LM BODY EXT NECK & STD NECK
- Zimmer Biomet VERSYS 6 INCH BEADED FC 12X160MM LM BODY EXT NECK
- Zimmer Biomet CPT 12/14 COCR, SIZE 0, STD
- Zimmer Biomet CPT 12/14 COCR REVISION SIZE 2 180MM
- Zimmer Biomet CPT 12/14 COCR SIZE 1 EXT
- Zimmer Biomet CPT 12/14 COCR Sz 2 Multiple Lot Numbers
- Zimmer Biomet CPT 12_14 COCR Sz 2 STD Multiple Lot Numbers
- Zimmer Biomet BIPOLAR METAL SHELL 48 MM OD
- Zimmer Biomet CPT 12/14 COCR SIZE 2 EXT
- Zimmer Biomet NEXGEN POROUS, HA/TCP, UNCEMENTED FEM & TIB BASEPLATE, SZ C thru G, LT & RT, MINUS
- Zimmer Biomet Nexgen Porous, HA/TCP, Uncemented Fem & Tib Baseplate, SZ C thru G, LT & RT, MINUS, MULTIPLE CAT #S
- Zimmer Biomet NEXGEN POROUS, UNCEMENTED FEMORAL AND TIB BASEPLATE, SZ E & F, LT
- Zimmer Biomet NEXGEN POROUS, HA_TCP, UNCEMENTED FEM & TIB BASEPLATE, SZ C thru G, LT&RT
- Zimmer NexGen LPS Option Fem Comps Sz C thru H
- Zimmer NexGen LPS Fem Comp Sz B thru G
- Zimmer NexGen LPS Fem Comp Sz G, Left
- Zimmer NexGen LPS Precoat Femoral Sz D & E
- Zimmer NEXGEN LPS Flex Precoat Fem Comp, Sz B thru G
- Zimmer NEXGEN LPS Flex Porous Fem Comp, Sz E
- Zimmer NexGen LPS Precoat Femoral Sizes D thru F
- Zimmer NexGen LPS Flex Option Fem Comp
- Zimmer NEXGEN LPS Flex GSF Option, SZ C thru G, LT & RT
- Zimmer NEXGEN LPS Flex GSF Porous Fem, SZ F, RT
- Zimmer NEXGEN CR Flex Option Femoral Minus, Sz C thru G
- Zimmer NEXGEN CR Flex Opt Femoral, Sz C thru G, LT & RT
- Zimmer NEXGEN CR Flex Porous Femoral
- Zimmer NexGen CR Flex GSF Precoat, SZ C thru G, LT & RT
- Zimmer The Natural Knee II Sys Cancellous Structured Titanium Porous Coating
- Zimmer Natural Knee Flex_NexGen CR Flex
- Zimmer Natural Knee Flex GSF NonPorous Fem
- Zimmer Natural Knee Flex GSF Porous Fem
- Zimmer NEXGEN Complete Knee Solution Legacy Posterior Stabilized, Sz H-Right
- Zimmer NEXGEN Complete Knee Solution Legacy Posterior Stabilized, LCCK, Sz C thru F
- Zimmer NEXGEN Complete Knee System Precoat Fem Comp
- Zimmer NEXGEN Knee System CR Option Fem Comp
- Zimmer NEXGEN Complete Knee Solution CR
- Zimmer NEXGEN Knee, SZ 4 thru 8, LT AND RT
- Zimmer PFJ Fem Cemented
- Zimmer PFJ Fem Cemented, SZ 4, RT
- Zimmer Unicomp Knee, SZ B thru F, RT & LT, MED & LAT
- Zimmer Unicomp Knee, RT and LT, SM, REG and LGE
- Zimmer Biomet Versys 6 inch Beaded Full Coat Plus Hip Prosthesis, multiple Catalog Numbers and Expiration Dates
- Zimmer Segmental System, ZSS Distal Femur, multiple Catalog Numbers and Expiration Dates
- DePuy Corail Coxa Vara HO Collared Stem, Sz9, Part No. 3L93709
- Depuy Corail HO Collarless Stem, Sz14, Part No. L20314
- Exactech Alteon Femoral Stem Collared, Sz5, Catalog #190-30-05
- Zimmer G7 Dual Mobility Liner, F-44 mm, Item #110024464
- Zimmer AGC Porous Patellar, Med, Model #150804
- Zimmer AGC Patella Porous, SM, Model #150802
- Zimmer Vanguard CR Tibial Bearing, 12×71/75mm, Item #183442
- Zimmer Vanguard CR Lipped Tibial Bearing, 10×71/75mm, Item #183540
- Biolox Delta Option Ceramic Heads, 32MM, Model #650-1056
- Ceramic Option Type 1 Taper Sleeve, -6, Model #650-1064
- Echo Bimetric Porous Femoral Hip System, 9×125, Model #192009
- OSS Distal Femoral LT ASSY, 19CM, Model #CP111828
- OSS Distal Femoral RT ASSY, 19CM, Model #CP111817
- OSS Cemented IM Stem 12×150, Model #150366
- Zimmer Biomet PPS RingLoc+ Acetabular Shell, SZ62, Model #11-116062
- Medacta GMK Knee System
2017 Class 2 Recalls
- An intermittent electrical problem that could lead to a loss of system power due to a loose screw connection
- Repicci II Tibial Components
- Zimmer Biomet Custom Polyethlylene Implants
- Endotoxin levels higher than process maximum limits were discovered in the identified polyethylene components.
- Multiple products including Active Articulation, Biolox Delta Option Head, RingLoc
Field Safety Notice
- Biomet UK issued a voluntary field safety corrective action; analysis identified a world wide occurrence of 0.12% of patients experiencing tibial plateau fractures.
- In a small number of cases, cracks that occur in the welds of the head of the mallet have resulted in the escape of some lead beads.
- Vanguard Total Knee System
- Zimmer Biomet RingLoc ArComXL Highly Crosslinked Polyethylene Liners
Instruments
- Exactech Logic Fit Tibial Tamp Head
- Exactech 1.5” Novation Calcar Planer Guide Tip
- Orthosize Templating Version 1.2.6 Echo Bi-Metric Hip Stem Digital Templates (Zimmer Biomet)
- Stryker Instruments Precision Match Head , Precision Round, MIS (minimally invasive surgery) product to remove osseous material during surgical cases. Multiple sizes.
- Handle that is used to hold surgical components during acetabular reaming in MAKO Total Hip Procedures
- Five AFMEA Riskk Control Measures are missing from the User Guides.
- Vanguard Total Knee, Punch Thru TRL Plates, 63mm, 67mm, 71mm, 75mm, 79mm
- Oxford Knee System Tibial Resector Body Tube & Guides
- Oxford Knee System Femoral Slap Hammer
- Left Medial Tibial Trial Tray Size A
- Left Medial Tibial Trial Tray Size B
- Left Medial Tibial Trial Tray Size C
- Left Medial Tibial Trial Tray Size D
- Left Medial Tibial Trial Tray Size E
- Left Medial Tibial Trial Tray Size F
- Phase 3 Tibial Template L Medial Size B
- Phase 3 Tibial Template L Medial Size C
- Phase 3 Tibial Template R Medial Size A
- Phase 3 Tibial Template R Medial Size B
- Phase 3 Tibial Template R Medial Size C
- Phase 3 Tibial Template R Medial Size D
- Right Medial Tibial Trial Tray Size A
- Right Medial Tibial Trial Tray Size B
- Right Medial Tibial Trial Tray Size C
- Right Medial Tibial Trial Tray Size D
- Right Medial Tibial Trial Tray Size E
- Right Medial Tibial Trial Tray Size F
- Oxford Unicompartmental Knee Phase 3 Shim Size 1
- Oxford Unicompartmental Knee Phase 3 Shim Size 2
- Oxford Unicompartmental Knee Phase 3 Shim Size 3
- Oxford Unicompartmental Knee Phase 3 Tibial Impactor
2016 Class 1 Recalls
DuPuy Synthes Power Tool System Battery Adaptor
Greatbatch Medical Standard Offset Cup Impactor – Inadequate Sterilization
2016 Class 2 Recalls
Posted April, 2016
The following Class II Recall Notifications from Zimmer Biomet include a variety of catalog and lot numbers. The reason, cause, and action for each recall can be found in each of the .pdf documents listed here for your reference. A link to the FDA website for each recall is also included at the bottom of each .pdf file. Please review the details to determine any impact to your cases.
The reason, cause, and action for the recalls listed below is as described by the FDA on its website:
Manufacturer Reason for Recall
As a result of the insufficient sealer calibration data for product packaged in firm’s Building II between August 2010 and April 2013. The affected products are sterile.
FDA Determined Cause 2
Zimmer Biomet sent an Urgent Medical Device Recall Letter, dated January 11, 2016, to Distributors, Sales Representatives, Operation Managers, and Risk Managers. The letter identified the product, the problem, and the action to be taken by the customer. Customers were instructed to:
- Review the notification and ensure that relevant personnel are aware of the contents.
- Assist your Zimmer Biomet sales representative with the quarantine of any affected product.
- Your Zimmer Biomet sales representative will remove the recalled product from your facility.
- Complete and return the attached Certificate of Acknowledgment form to [email protected].
- If after reviewing this notification you have further questions or concerns please call
Customer Service at 1-800-348-2759, or contact your Zimmer Biomet Sales Representative
- Zimmer Packaging Recall
- NexGEN System REF 5980-37-01 Tibial Component Size Stemmed 3 Precoat, and REF 5986-37-01 Nonaugmentable Tibial Component Option CR/PS/LPS Size Stemmed 3
- SYNTHES; 13.5MM Medullary Reamer Head; 352.135 NON STERILE
November, 2016
- Stryker LFIT Anatomic V40 Femoral Head
October, 2016
- Stryker Sagittal Blade 18.0X0.97X90MM
- Zimmer Gender Solutions Patellofemoral Joint Prosthesis Milling Handpiece
September, 2016
- Stryker Orthopaedics Patella Assembly Instrument (Scorpio Patella Assembly)
August, 2016
- Stryker Orthopaedics Specialty Triathlon Tibial Alignment Handle with Secondary Lock
- (Stryker) Exeter Rasp/Trial Introducer/Extractor Handle part of the Exeter Femoral Hip System
- Stryker Orthopaedics Trident Constrainer Liner Inserter/Impactor Tip, 22mm, 28mm, and 32mm (sterilization issue)
- Zimmer Persona Trabecular Metal Tibial Plate Instruments and Modular Brackets
- Prelude Patella Femoral Resurfacing Knee System Instrumentation
- Restoris PST Offset Shell Impactor
- Robotic Arm Interactive Orthopedic System (MAKO)
2015 Class 1 Recalls
Zimmer M/L Taper with Kinectiv Technology Prosthesis Femoral Stems and Necks Higher than Expected Levels of Manufacturing Residues
Persona Trabecular Metal Tibial Plate / Persona TM Tibia-Prosthesis, Knee, Patello/Femorotibial, Semi-Constrained, Uncemented, Porous, Coated, Polymer/Metal/Polymer
MicroPort Orthopedics Inc., PROFEMUR Neck Varus/Valgus CoCr 8 Degree
2015 Class ll Recalls
LCS COMPLETE RPS Femoral Implants, various sizes and orientation. Knee prosthesis component for orthopedic surgery
LCS COMPLETE RPS inserts various sizes. Knee prosthesis component for orthopedic surgery
Stryker Trident 10 degrees x 3 Insert 36 MM
Stryker Triathlon x3 Tibial Bearing Insert
Stryker Triathlon PS x3 Tibial Insert, No 3 Triatholon TS Plus Tibial Insert x 3 Poly 19 mm
Stryker Orthopaedics Tapers LFIT V40 Vitallium femoral heads. hip prosthesis component
NexGen System, Complete Knee Solution; Reference Number 5980-37-01; Tibial Component, Precoat, Stemmed Size 3
Stryker Orthopaedics Duracon Flat Tibial Wedge Cemented use only Sterile
Instruments
Stryker MIS Modular Distal Capture Triathlon MIS Instruments
SSS Reprocessed Zimmer, Synthes, Stryker and Linvatec Drill Bits and Blades
Zimmer UNIVERSAL Power System Loaner and Modular Electric/Battery Double Trigger Handpiece. Rx only Made in Switzerland

- En Español

- Medical Devices
- Radiation-Emitting Products
- Vaccines, Blood & Biologics
- Animal & Veterinary
- Tobacco Products
Class 2 Device Recall Journey BCS Knee System
This website has been translated to Spanish from English, and is updated often. It is possible that some links will connect you to content only available in English or some of the words on the page will appear in English until translation has been completed (usually within 24 hours). We appreciate your patience with the translation process. In the case of any discrepancy in meaning, the English version is considered official. Thank you for visiting esp.fda.gov/tabaco.
ORTHOPAEDICS
Journey ◊ ii total knee arthroplasty.
Total knee arthroplasty patients report unmet levels of satisfaction, particularly for more active or demanding activities. 1,2 The JOURNEY II System is designed to help patients rediscover their normal through a smoother recovery, *3,4 improved function *4-8 and higher patient satisfaction *2,4-6
Normal shapes. Normal position. Normal motion
Reproduction of optimal kinematic patterns during TKA could be instrumental in improving patient satisfaction. 11 The solution to providing patients with better overall satisfaction and functionality is to design an implant as close to the normal knee as possible.
The JOURNEY II System has been shown to restore anatomical shape, position and motion. 9,10,12-14 This anatomical restoration can provide superior clinical outcomes that can lead to high patient satisfaction. *3-5,8,15
Product Features
Journey ii tka video: rediscover normal, show videos, reference materials, medical education, related products.
* Compared to non-JOURNEY II knees.
This design rationale is for informational and educational purposes only. It is not intended to serve as medical advice. It is the responsibility of treating physicians to determine and utilise the appropriate products and techniques according to their own clinical judgment for each of their patients.
For detailed product information, including indications for use, contraindications, effects, precautions and warnings, please consult the product’s Instructions for Use (IFU) prior to use.
- Scott CEH, et al. J Bone Joint Surg Am. 2010;92-B(9):1253-1258.
- Noble P.C, et al. Clin Orthop Relat Res 2012;470(1):20-32
- Mayman DJ, et al. Poster presented at: ISPOR Symposium;19-23 May, 2018; Baltimore, Maryland, USA
- Nodzo SR, et al. Techniques in Orthopaedics. 2018;33(1):37-41.
- Murakami K, et al. Int Orthop. 2018;42(11):2573-2581.
- Di Benedetto P, et al. Acta Biomed 2019; Vol. 90, Supplement 12: 91-97.
- Kosse NM, et al. Poster presented at: 2nd World Arthroplasty Congress;19-21 April, 2018; Rome, Italy.
- Takubo A, et al. J Knee Surg. 2017;30(7):725-729.
- Grieco T, et al. J Arthroplasty. 2018;33(2):565-571.
- Smith LA, et al. J Arthroplasty. 2021;36:1445-1454.
- Van Onsem S, et al. Clin Orthop Relat Res (2020) 478:255-263.
- Iriuchishima T, et al. J Knee Surg. 2018;31(6):568-572.
- Murakami K, et al. J Orthop. 2018;15(2):650-654.
- Carpenter RD, et al. Knee. 2009;16(5):332-336.
- Noble PC, et al. Clin Orthop Relat Res. 2005(431):157-165.
- Kaneko T, et al. J Orthop. 2017;14(1):201-206.
- Brilhault J, et al. Knee. 2010;17(2):148-151.
- Catani F, et al. J Orthop Res. 2009;27(12):1569-1575.
- Hyodo K, et al. Arthroplasty Today. 2020;6(3):338-342.
- Hada M, et al. Knee Surg Sports Traumatol Arthrosc. 2018;26(6):1709-1716.
- Smith+Nephew 2012. Internal report. JRN2 KneeSim Analysis Memo.

IMAGES
VIDEO
COMMENTS
Smith & Nephew's first-generation knee replacement device was recalled on Oct. 1 as the Food and Drug Administration announced the Class 2 device recall online, following a series of research and tests regarding the high failure rate of the device.. Smith & Nephew's JOURNEY Bi-Cruciate Stabilized (BCS) Knee system was the first-generation version of the JOURNEY II BCS which replaced the ...
The AAOS Device Recall Dashboard provides orthopaedic surgeons with timely recall information that will protect the health and well-being of their patients. ... Smith & Nephew, Inc. JOURNEY II BCS: ... Knee insert and acetabular system parts were swapped within inner pack prior to the product being sealed within the tray, which could result in ...
Possible first generation Smith & Nephew Journey BCS knee implant complications for which you could receive compensation may include: ... Knee Implant Recall. Smith & Nephew voluntarily recalled its first generation Journey BCS Knee System on June 13, 2018. The U.S. Food and Drug Administration later categorized this recall as Class 2.
JOURNEY II BCS CONSTRAINED ARTICULAR INSERT Size 5-6, 10 MM Left, REF 74029262; knee prosthesis: Code Information: UDI/DI 00885556176467, Batch Number 22BM17564: Recalling Firm/ Manufacturer: Smith & Nephew, Inc. 1450 E Brooks Rd Memphis TN 38116-1804: For Additional Information Contact: David Snyder 978-749-1440: Manufacturer Reason for Recall
JOURNEY II UNI XLPE TIBIA INSERT MEDIAL SZ 7-8 8MM. Code Information. Catalog Number: 74026178; UDI/DI: 00885556677209; Batch Number: 22CAP0038R; Lot Number: 22CAP0038R. Recalling Firm/. Manufacturer. Smith & Nephew, Inc. 1450 E Brooks Rd. Memphis TN 38116-1804. For Additional Information Contact.
Smith & Nephew Journey I BCS Knee Recall. In June 2018, Smith & Nephew voluntarily issued a recall for the Journey I BCS Knee. In the Urgent Field Safety Notice the company released, it explains that the knee was found to have a revision rate one and a half times higher than similar knee implants. The company further stated that recipients of ...
Smith & Nephew (S&N) has announced several recalls for the Journey knee replacement. The problem with some implants is a defective tibial baseplate or femoral implant. If these components break, patients may develop severe pain, instability, and require revision surgery. Journey BCS Knee Recall in Australia
Recall # Z-0158-2024 Smith & Nephew - JOURNEY II BCS CONSTRAINED ARTICULAR INSERT Size 5-6, 10 MM Left. JOURNEY II BCS CONSTRAINED ARTICULAR INSERT Size 5-6, 10 MM Left, REF 74029262; knee prosthesis - The JRNY II BCS XLPE ART ISRT SZ 5-6 LT 10MM was laser etched, labeled and packaged as a JRNY II BCS CNSTRD ART ISRT 5-6 LT 10MM.
Class 2 Device Recall Journey BCS Knee System: Date Initiated by Firm: June 13, 2018: Date Posted: October 01, 2018: Recall Status 1: Open 3, Classified: Recall Number: Z-0002-2019: Recall Event ID: 80313: ... Smith & Nephew, Inc. 1450 E Brooks Rd Memphis TN 38116-1804: For Additional Information Contact:
Total knee arthroplasty patients report unmet levels of satisfaction, particularly for more active or demanding activities. 1,2 The JOURNEY II System is designed to help patients rediscover their normal through a smoother recovery, *3,4 improved function *4-8 and higher patient satisfaction *2,4-6. Normal shapes. Normal position.