Aller au contenu | Navigation | Accès directs | Connexion
Personnel hébergé

M. Thomas Desmidt
Coordonnées.
10 Boulevard Tonnellé 37032 TOURS CEDEX 1
Discipline(s)
- accéder au contenu
- accéder au menu principal
- contactez-nous
- Newsletters
- Transparence
- Nous contacter
- Accueil France Info
- Vrai ou faux
- Elections législatives 2024
- Résultats du bac 2024
- L'actu pour les jeunes
- Une info transparente
- Faits-divers
- Environnement
- Éco / conso
- Sciences & technologies
- France 3 régions
- Outremer la 1ère
- Radio france
- Guerre entre le hamas et israël
- Guerre en ukraine
- Crise climatique
- Donald trump
- Présidentielle américaine 2024
- Voir plus de sujets monde
- Asie-Pacifique
- Etats Unis d'Amérique
- Proche-orient
- Story Killers
- Conflit dans le Haut-Karabakh
- Guerre entre Israël et le Hamas
- Inondations meurtrières en Libye
- Séisme au Maroc
- Voir plus de sujets europe
- Guerre en Ukraine
- Sommet de l'UE
- Royaume-Uni
- Enlèvements
- Justice – Procès
- Incendies de forêt
- Mort de Nahel à Nanterre
- Disparition de Delphine Jubillar
- Attaque au couteau à Annecy
- Procès de l’attentat de Magnanville
- Disparition du petit Emile
- Incendie meurtrier à Wintzenheim
- Dissolution de l'assemblée nationale
- Emmanuel macron
- Gouvernement de gabriel attal
- Voir plus de sujets politique
- Élections européennes 2024
- Gouvernement de Gabriel Attal
- Assemblée nationale
- Emmanuel Macron
- Réforme des retraites 2023
- Les Républicains
- Renaissance
- Rassemblement national
- Parti socialiste
- La France insoumise
- Les Ecologistes - EELV
- Election présidentielle
- Toutes les élections
- Manifestations propalestiniennes
- Calendrier des vacances scolaires
- Immigration
- Violences sexuelles
- Voir plus de sujets société
- Egalité femmes-hommes
- Maltraitance dans les Ehpad
- Droits des femmes
- Féminicides
- Prostitution
- Résultats Bac
- Résultats Brevet
- Résultats Bts
- Résultats Cap
- Harcèlement scolaire
- Voir plus de sujets environnement
- Convention citoyenne sur le climat
- Biodiversité
- Pollution de l'air
- Le tableau de bord du réchauffement climatique
- Nos réponses à vos questions sur le climat
- Empreinte carbone
- Montée des eaux
- Greta Thunberg
- Carte des restrictions d'eau
- Tableau de bord des nappes phréatiques
- Calculez votre empreinte carbone
- Calculez l'impact carbone de vos déplacements
- Trouvez des fruits et légumes de saison
- Triez mieux vos déchets
- Tour de France 2024
- Tour de france 2024
- Jeux olympiques de paris 2024
- Voir plus de sujets sport
- Jeux paralympiques
- Roland-Garros
- Tour de France
- Coupe du monde de foot
- Mondiaux d'athlétisme
- Coupe du monde de rugby
- La culture à l'heure des jeux 2024
- Haute couture
- Voir plus de sujets culture
- Festival de Cannes 2023
- Oscars 2024
- Victoires de la musique 2024
- Festival du livre de Paris 2024
- Les Molières
- Fête de la musique 2024
- Rock en Seine 2024
- Festivals d'été
- France télévisions
- Voir plus de sujets éco / conso
- Pouvoir d'achat
- Impôts - Fiscalité
- Entreprises
- Auto-entrepreneurs
- Aéronautique
- Budget de la France
- Soldes d'hiver 2024
- Vaccin contre le Covid-19
- La crise de l’hôpital public
- Biologie - Génétique
- Alimentation
- Lutte contre le tabagisme
- Politique de santé
- Psycho - Bien-être
- Sport et santé
- Professions médicales
- Le tabou de la santé mentale
- Météorites en Russie
- Astéroïde 2012 DA14
- Steak in-vitro
- Boson de Higgs
- Solar Impulse
- Homo naledi
- Intempéries
- Météo France
- Météo Paris
- Météo Marseille
- Actualités météo
- Mots fléchés
- Mots croisés
- Mots mystères
- France tv & vous
- France tv le club
- France tv lab
- Les Médiateurs
- France Inter
- France Bleu
- France Culture
- France Musique
- Maison de la Radio et de la Musique
- La Médiatrice
Santé : du gaz hilarant pour traiter la dépression, l'étonnante découverte d'une équipe de chercheurs
Une équipe de chercheurs du CHU de Tours a publié le 17 août un article dans la revue Molecular Psychiatry, détaillant les effets sur le cerveau d'un mélange de dioxygène et de protoxyde d'azote, dans le cadre du traitement de syndrôme dépressif.
Une " première mondiale ", made in Indre-et-Loire. Une équipe de l'université de Tours vient peut-être de participer à une révolution dans le traitement médical de la dépression. Dans un article publié dans la revue Molecular Psychiatry le 17 août, des chercheurs montrent les effets sur le cerveau de l'exposition de patients dépressifs à du gaz hilarant.
Des effets " évidents, qui nous confirment dans nos résultats ", explique à France 3 le professeur Thomas Desmidt. Coauteur de l'article, il est professeur de psychiatrie à la faculté de médecine de Tours, médecin au CHU de Tours et chercheur à l'Inserm, l'institut national de la santé et de la recherche médicale. En se fondant sur ces résultats, le professeur estime que le gaz hilarant pourrait " faire partie de la nouvelle génération d'antidépresseurs ".
Disparition des syndromes dépressifs
Depuis plusieurs années déjà, les scientifiques disposent de données " qui laissent penser que le gaz hilarant, sous sa forme médicale utilisée pour le traitement des douleurs, pourrait avoir un effet antidépresseur ". L'équipe tourangelle a souhaité identifier ce qui se passe au sein du cerveau après exposition au produit, pour tenter d'en expliquer les effets.
L'étude a été menée sur un petit échantillon de 30 femmes volontaires, âgées de 25 à 50 ans, au sein du CHU de Tours. Toutes ont passé une IRM, puis ont été soumises à une séance d'exposition à du gaz hilarant d'une heure, avant de passer une nouvelle IRM. Près de la moitié de ces femmes a répondu au traitement, avec une " absence quasiment totale des symptômes dépressifs les semaines suivantes, voire plusieurs mois après pour certaines patientes ".
Comment ça marche
Le fonctionnement neurologique des symptômes dépressifs est bien connu : " Le cerveau fonctionne sur le mode du réseau , explique Thomas Desmidt. Différentes parties s'activent de façon synchrone, pour les fonctions cognitives classiques chez une personne non-dépressive. Mais cette activation synchrone peut produire des symptômes de la dépression, comme les ruminations, la négativité, les idées noires... "
Concrètement, les scientifiques de Tours, en partenariat avec une équipe américaine de l'université de Pittsburgh, ont pu constater un " soulagement d'une certaine hyperactivation de certaines zones du cerveau associées à des symptômes dépressifs " après exposition au gaz hilarant, précise le professeur.
Mieux comprendre ce mécanisme ouvre des portes dans le futur. De nouvelles études sont en cours au CHU de Tours, et pourraient " se terminer d'ici deux ans ", selon Thomas Desmidt.
Vers une utilisation généralisée en médecine ?
Si bien que, " si les résultats sont probants, on pourrait commencer à utiliser le gaz hilarant en soins courants dans quatre ou cinq ans ", espère-t-il. Sous réserve de validation par les autorités du médicament.
Si sa mise en circulation est très attendue, c'est que le gaz hilarant possède un avantage très concret : son efficacité à court terme, dès les heures suivant une exposition au produit.
Les antidépresseurs ont souvent un effet retardé. Il leur faut quelques semaines, voire quelques mois, pour avoir une efficacité patente... quand ils ont une efficacité. Thomas Desmidt, professeur de psychologie, Fac de médecine et CHU de Tours
Car, selon lui, " environ 30% des patients sont résistants aux antidépresseurs conventionnels, aux méthodes médicamenteuses et à la psychothérapie ".
Il ne faut pas prendre du "proto" pour soigner la dépression
Le produit utilisé par l'équipe tourangelle, qualifié de gaz hilarant, porte le doux nom scientifique de "mélange équimolaire d'oxygène et de protoxyde d'azote", ou MEOPA. Soit un mix à quantités égales de dioxygène (le même que l'on trouve dans l'air) et de protoxyde d'azote (le même que l'on trouve dans les siphons de chantilly).
Ce dernier est par ailleurs utilisé, depuis plusieurs années, de manière récréative sous sa forme pure, avec des effets graves " au niveau pulmonaire à court terme, et neurologique à long terme ", avertit Thomas Desmidt. Le protoxyde d'azote est d'ailleurs interdit à la vente pour les mineurs depuis 2021, et est contre-indiqué en milieu médical. Le MEOPA est, de son côté, un produit médical, utilisé dans un cadre précis et surveillé.
Pour aller plus loin
- psychologie
- Indre-et-Loire
- Centre-Val de Loire

- copier le lien https://france3-regions.francetvinfo.fr/centre-val-de-loire/indre-loire/tours/sante-du-gaz-hilarant-pour-traiter-la-depression-l-etonnante-decouverte-d-une-equipe-de-chercheurs-2835854.html
- Bourg-en-Bresse
- Clermont-Ferrand
- Le Puy-En-Velay
- Saint-Etienne
- Haute-Loire
- Haute-Savoie
- Puy-de-Dôme
- Toute la région
- Chalon-sur-Saône
- Lons le Saunier
- Luxeuil Les Bains
- Montbéliard
- Côte-d'Or
- Haute-Saône
- Saône-et-Loire
- Territoire de Belfort
- Saint-Brieuc
- Côtes-d'Armor
- Ille-et-Vilaine
- Châteauroux
- Eure-et-Loir
- Loir-et-Cher
- Corse-du-Sud
- Haute-Corse
- Châlons-en-Champagne
- Charleville-Mézières
- Saint-Dizier
- Haute-Marne
- Meurthe-et-Moselle
- Boulogne-sur-Mer
- Saint-Quentin
- Valenciennes
- Pas-de-Calais
- Cherbourg-En-Cotentin
- Seine-Maritime
- Châtellerault
- La Rochelle
- Mont-de-Marsan
- Sarlat-la-Canéda
- Charente-Maritime
- Deux-Sèvres
- Haute-Vienne
- Lot-et-Garonne
- Pyrénées-Atlantiques
- Montpellier
- Haute-Garonne
- Hautes-Pyrénées
- Pyrénées-Orientales
- Tarn-et-Garonne
- Grand Paris
- Saint-Denis
- Hauts-de-Seine
- Seine-et-Marne
- Seine-Saint-Denis
- Val-de-Marne
- Val-d'Oise
- La Roche-sur-Yon
- Les Sables-d'Olonne
- Saint-Nazaire
- Loire-Atlantique
- Maine-et-Loire
- Aix-en-Provence
- Digne-les-Bains
- Fréjus Et Saint-Raphaël
- Saint-Tropez
- Alpes-de-Haute-Provence
- Alpes-Maritimes
- Bouches-du-Rhône
- Hautes-Alpes
- Toute la France
Dr THOMAS DESMIDT
Chru bretonneau - tours.
2 Boulevard TONNELLE 37200 Tours
Informations
Horaires et contact.
02 47 47 47 47
Vous êtes Dr THOMAS DESMIDT ? Modifier vos informations
Vous êtes professionnel de santé ?
Découvrez l'agenda en ligne et la téléconsultation par Maiia
Besoin d'aide ?
Visitez notre centre de support ou contactez-nous !
Maiia - © 2024 Tous droits réservés
Version 1.205.0.187
Les professionnels de santé ayant souscrit à la prise de rendez-vous en ligne apparaissent en priorité dans les pages de recherche et d'annuaire.

- Dr Desmidt Thomas
Psychiatre Tours
- Lemedecin.fr
- Indre-et-loire
Présentation
Docteur DESMIDT THOMAS est psychiatre à Tours, CHRU BRETONNEAU - TOURS est au 2 Boulevard TONNELLE à Tours dans le 37044.
Tarifs et remboursements
Conventionnement Non renseigné
Carte vitale Non renseigné
Informations pratiques
Adresse Dr Desmidt Thomas 2 Boulevard TONNELLE 37044 Tours
Langue parlée français
Accès handicapé Non renseigné
Praticiens au sein de la même structure

Praticien(s) à la même adresse

Informations administratives
- Consultation au 2 Boulevard TONNELLE à Tours dans le 37044
- DR LAFFON MARC, anesthésiste réanimateur
- DR BOUVART LAURA, pédiatre
- DR GARGOT THOMAS, psychiatrie-option-enfant-et-adolescent
- DR VOYER CARINE, cardiologue
- DR MAROT YVES, médecin généraliste
- DR DESTRIEUX CHRISTOPHE, neurochirurgien
- DR CHAPET SOPHIE, onco-radiothérapeute
- DR COUDRIOU CHARLES, anesthésiste réanimateur
- DR MANGIN JEAN-CHRISTOPHE, anesthésiste réanimateur
- DR MICHEL NATACHA, gériatre

Si votre médecin traitant n'est pas disponible dans un délai compatible avec votre état de santé : Consultation remboursable si le médecin téléconsultant appartient à une organisation coordonnée de votre territoire.
Si vous n’avez pas de médecin traitant : Consultation remboursable si vous résidez dans une zone avec une offre de soins faible et dépourvue d'organisation territoriale coordonnée. Si, en tant que patient, votre cas ne correspond pas à une des 3 options citées ci-dessus, cela signifie que votre consultation ne sera pas prise en charge et ne donnera pas lieu à l'émission d'une feuille de soins. Pour bénéficier d’un remboursement, nous vous invitons à transmettre la note d’honoraire émise à l’issue de votre téléconsultation à votre mutuelle. En fonction de votre contrat, une prise en charge pourrait être prévue. LEMEDECIN.FR n’est pas un offreur de soins mais un opérateur de mise en relation entre un patient et un professionnel de santé. Aucun lien de subordination n’existe entre les médecins libéraux adhérents de la plateforme et LEMEDECIN.FR. L’émission d’ordonnances, d’arrêt de travail, de feuilles de soins ou de compte-rendus médicaux demeurent de la seule responsabilité du professionnel de santé et en aucun cas, LEMEDECIN.FR ne peut s’immiscer dans la relation entre un patient et son praticien, laquelle fait spécifiquement l’objet d’une protection par l’intermédiaire du secret médical. Un outil est mis à votre disposition pour tester votre éligibilité à une prise en charge par l’assurance maladie.
- Je confirme que le praticien recherché n’est pas disponible dans un délai compatible avec mon état de santé
- J'accepte une consultation vidéo avec un autre praticien
- Le parcours de soins coordonnés avec votre praticien habituel reste à privilégier

en un clin d'oeil , prenez rdv en ligne avec un médecin

- Toutes les spécialités
- Médecin - Psychiatre à Tours
- Dr DESMIDT Thomas
- Informations
Adresse d'exercice de Dr DESMIDT Thomas
Chru bretonneau - tours.
- 2 Boulevard Tonnelle, 37044 Tours
informations complémentaires
- n° SIREN : 263700189
- n° FINESS : 370000861
- VOIR LES COORDONNÉES DE CE PRATICIEN (Téléphone & Mail)
Présentation : Psychiatre
Dr Thomas DESMIDT (Psychiatre) exerce son activité à 1 adresse : - CHRU BRETONNEAU - TOURS : 2 Boulevard Tonnelle, 37044 Tours
Moyens de Transports les plus proches
- Bretonneau (Arrêt de bus)
- Desmoulins (Arrêt de bus)
- Tonnellé (Arrêt de bus)
- Ste Anne (Arrêt de bus)
- Walvein (Arrêt de bus)
- MAME (Arrêt de bus)
Réseau de Dr DESMIDT Thomas
- Catherine Marambaud (Masseur-Kinésithérapeute Tours)
- Dr Anne Girault-lataste (Médecin Néphrologie Tours)
- Claire Besnainou (Masseur-Kinésithérapeute Tours)
- Dr Roland Crenn (Médecin Anesthesie-réanimation Tours)
- Dr Annie-pierre Jonville-bera (Médecin Santé publique et médecine sociale Tours)
- Marie-helene Estienne (Pharmacien Tours)
- Dr Lionel Homer (Médecin Gynéco-obstétrique et Gynéco médicale option Gynéco-obst Tours)
- Dr Antoine Bouissou (Médecin Pédiatrie Tours)
- Hortense Touchard (Masseur-Kinésithérapeute Tours)
- Dr Olivier Pincon (Médecin Qualifié en Médecine Générale Tours)
- Coline Planchaud (Sage-Femme Tours)
- Dr Marie-charlotte Bernard (Médecin Biologie médicale Tours)
- Dr Chantal Cendrie (Médecin Médecine Générale Tours)
- Dr Bertrand De Toffol (Médecin Neuro-psychiatrie Tours)
- Dr Jorge Domenech (Médecin Biologie médicale Tours)
- Monique Lavaud (Sage-Femme Tours)
- Romain Pouponneau (Sage-Femme Tours)
- Dr Elisabeth De Charry-diot (Médecin Médecine interne Tours)
- Dr Marion Stacoffe (Médecin Oncologie option médicale Tours)
- Dr Catherine Denis (Médecin Cardiologie et maladies vasculaires Tours)
- Dr Emmanuel Simon (Médecin Gynécologie-obstétrique Tours)
- Dr Carl Semaan (Médecin Cardiologie et maladies vasculaires Tours)
- Dr Catherine Barbieux (Médecin Médecine du travail Tours)
- Celia Meireles De Carvalho (Sage-Femme Tours)
- Dr Gilles Body (Médecin Gynécologie médicale et obstétrique Tours)
- Dr Ruxandra Gautard (Médecin Néphrologie Tours)
- Dr Dominique Sirinelli (Médecin Radio-diagnostic Tours)
- Dr Dominique Saillant (Médecin Pédiatrie Tours)
- Dr Valerie Gissot (Médecin Médecine interne Tours)
- Lydie Faillenet (Sage-Femme Tours)
- Dr Nicolas Guyot (Médecin Spécialiste en Médecine Générale Tours)
- Elodie Igout (Sage-Femme Tours)
- Nejla Massoumi (Masseur-Kinésithérapeute Tours)
- Carole Leprince (Sage-Femme Tours)
- Dr Benjamin Anon (Médecin Gastro-entérologie et hépatologie Tours)
- Dr Laurianne Drieu La Rochelle (Médecin Hématologie Tours)
- Dr Viviane Demoussy (Médecin Anesthesie-réanimation Tours)
- Dr Gaelle Derot (Médecin Radio-diagnostic Tours)
- Dr Amandine Loubiere (Médecin Oncologie option radiothérapie Tours)
- Dr Patrice Diot (Médecin Pneumologie Tours)
- Dr Virginie Andre-soibinet (Pharmacien Tours)
- Dr Julien Praline (Médecin Neurologie Tours)
- Dr Anne Teissedre (Pharmacien Tours)
- Claire Guillot (Sage-Femme Tours)
- Celine Marechal-guyot (Sage-Femme Tours)
- Dr Ilyess Zemmoura (Médecin Neuro-chirurgie Tours)
- Dr Adeline Boudet (Pharmacien Tours)
- Dr Thibault Moles (Médecin Néphrologie Tours)
- Dr Ammar El Ameen (Médecin Ophtalmologie Tours)
- Dr Marion Campana (Médecin Pneumologie Tours)
- Manon Cotret (Sage-Femme Tours)
- Dr Isabelle Bonnaud (Médecin Neurologie Tours)
- Dr Frederique Bonnet-brilhault (Médecin Psychiatrie Tours)
- Sophie Maury (Sage-Femme Tours)
- Philippe Ricotier (Masseur-Kinésithérapeute Tours)
- Dr Arnaud De Luca (Médecin Pédiatrie Tours)
- Dr Julie Magnant (Médecin Médecine interne Tours)
- Agnes Pons (Sage-Femme Tours)
- Dr Guy-noel Teinturier (Médecin Qualifié en Médecine Générale Tours)
- Dr Emmanuel Rusch (Médecin Santé publique et médecine sociale Tours)
- Dr Regis Labbe (Médecin Qualifié en Médecine Générale Tours)
- Dr Guillaume Gras (Médecin Spécialiste en Médecine Générale Tours)
- Dr Laetitia Bodet-contentin (Médecin Médecine intensive-réanimation Tours)
- Dr Patrick Lecerf (Médecin O.R.L et chirurgie cervico faciale Tours)
- Dr Christian Lamotte (Médecin Anesthesie-réanimation Tours)
- Dr Benjamin Faivre D'arcier (Médecin Chirurgie urologique Tours)
- Dr Bernard Desveaux (Médecin Cardiologie et maladies vasculaires Tours)
- Dr Frederic Loreille (Médecin Chirurgie générale Tours)
- Pr Alain Goudeau (Médecin Qualifié en Médecine Générale Tours)
- Dr Elisabeth Blin-zbiegiel (Médecin Spécialiste en Médecine Générale Tours)
- Dr Armelle Vinceneux (Médecin Oncologie option médicale Tours)
- Dr Anne Marie Bernard (Médecin Anesthesie-réanimation Tours)
- Dr Laurent Mereghetti (Médecin Biologie médicale Tours)
- Dr Fatiha Chabab (Médecin Pédiatrie Tours)
- Dr Eric Fournier (Médecin Anesthesie-réanimation Tours)
- Marie-camille Grenier (Sage-Femme Tours)
- Dr Philippe Lanotte (Pharmacien Tours)
- Arya Delgeon (Sage-Femme Tours)
- Juliette Meslet (Masseur-Kinésithérapeute Tours)
- Joelle Dabilly (Sage-Femme Tours)
- Dr Ken Haguenoer (Médecin Santé publique et médecine sociale Tours)
- Dr Helene Champion (Médecin Endocrinologie et métabolisme Tours)
- Dr Sandra Aymeric (Médecin Santé publique et médecine sociale Tours)
- Vanessa Fenelon (Sage-Femme Tours)
- Dr Michel Neny (Médecin Qualifié en Médecine Générale Tours)
- Dr Dominique Perrotin (Médecin Médecine interne Tours)
- Dr Guillaume Brachet (Pharmacien Tours)
- Dr Anna-chloe Balageas (Médecin Neurologie Tours)
- Dr Maelle Dejobert (Médecin Radio-diagnostic Tours)
- Dr Adrien Lemaignen (Médecin Médecine interne Tours)
- Dr Julie Balestra (Médecin Pédiatrie Tours)
- Dr Laura Vrignaud (Pharmacien Tours)
- Dr Victor Massot (Pharmacien Tours)
- Dr Catherine Maffre (Médecin Psychiatrie Tours)
- Dr Benedicte Mille-zemmoura (Médecin Anesthesie-réanimation Tours)
- Dr Ekaterina Petrova-berriot (Médecin Anesthesie-réanimation Tours)
- Dr Isabelle Mortemousque (Médecin Génétique médicale Tours)
- Carine Da Silva (Sage-Femme Tours)
- Viviane Himily (Sage-Femme Tours)
- Dr Louise Mennetrey (Médecin Qualifié en Médecine Générale Tours)
- Dr Marie-laure Couet (Médecin Gynécologie médicale et obstétrique Tours)
- Dr Laure Monleon (Médecin Chirurgie générale Tours)
- Dr Nicolas Ballon (Médecin Psychiatrie Tours)
- Dr Carine Voyer (Médecin Cardiologie et maladies vasculaires Tours)
- Dr Juliette Buraschi (Médecin Radio-diagnostic Tours)
- Dr Bernadette Le Noach (Médecin Qualifié en Médecine Générale Tours)
- Dr Cecilia Rousselot-denis (Médecin Anatomie et cytologie pathologiques Tours)
- Dr Beatrice Scotto (Médecin Radio-diagnostic Tours)
- Emilie Carre (Sage-Femme Tours)
- Christine Pate (Sage-Femme Tours)
- Sophie Pomes (Sage-Femme Tours)
- Dr Christine Collet (Pharmacien Tours)
- Philippe Pontoizeau (Masseur-Kinésithérapeute Tours)
- Dr Olivier Haillot (Médecin Chirurgie urologique Tours)
- Dr Jessica Rizk (Médecin Anesthesie-réanimation Tours)
- Dr Laurent Machet (Médecin Dermatologie et vénéréologie Tours)
- Dr Jacky Laulan (Médecin Chirurgie orthopédique et traumatologie Tours)
- Dr Antoine Schmitt (Médecin Chirurgie générale Tours)
- Dr Eirini Papadaki (Médecin Hématologie Tours)
- Dr Stephanie Roullier (Médecin Spécialiste en Médecine Générale Tours)
- Dr Guillaume Vandermeer (Médecin Ophtalmologie Tours)
- Dr Yann Venel (Médecin Médecine nucléaire Tours)
- Dr Jean-marc Serekian (Médecin Anesthesie-réanimation Tours)
- Cyril Knecht (Masseur-Kinésithérapeute Tours)
- Soline Bonnet (Sage-Femme Tours)
- Corinne Rocher (Sage-Femme Tours)
- Dr Philippe Corcia (Médecin Neurologie Tours)
- Dr Marie-sara Marchand (Pharmacien Tours)
- Sylvie Baumard (Sage-Femme Tours)
- Dr Charles-edouard Rouf (Médecin O.R.L et chirurgie cervico faciale Tours)
- Dr Chloé Plichon (Pharmacien Tours)
- Dr Denis Garot (Médecin Médecine intensive-réanimation Tours)
- Dr Sonia Mitreski (Pharmacien Tours)
- Sabine Lefeuvre (Sage-Femme Tours)
- Dr Martine Bidault (Médecin Médecine du travail Tours)
- Isabelle Boidron-balligand (Sage-Femme Tours)
- Dr Camille Dorbeau (Médecin O.R.L et chirurgie cervico faciale Tours)
- Dr Stephane Beltran (Médecin Neurologie Tours)
- Dr Francois Maillot (Médecin Médecine interne Tours)
- Claire Perrin (Sage-Femme Tours)
- Dr Joelle Bleuet (Médecin Gériatrie Tours)
- Dr Nathalie Trignol-viguier (Médecin Qualifié en Médecine Générale Tours)
- Dr Annie-laure Suc (Médecin Pédiatrie Tours)
- Clement Elphege (Sage-Femme Tours)
- Dr Fanny Borrelly (Médecin Médecine nucléaire Tours)
- Dr Thomas Desmidt (Médecin Psychiatrie Tours)
- Laure Bouffeteau (Sage-Femme Tours)
- Dr Lauranne Rossard (Médecin Gynécologie-obstétrique Tours)
- Dr Thomas Hebert (Médecin Gynécologie-obstétrique Tours)
- Dr Bernadette Berneron (Médecin Médecine du travail Tours)
- Dr Chantal Barin (Pharmacien Tours)
- Dr Sarah Gley-tekaya (Médecin Qualifié en Médecine Générale Tours)
- Dr Caroline Prunier- Aesch (Médecin Médecine nucléaire Tours)
- Nathalie Landier (Sage-Femme Tours)
- Dr Lotfi Benboubker (Médecin Oncologie option médicale Tours)
- Dr Hubert Coispeau (Médecin Qualifié en Médecine Générale Tours)
- Dr Maud Francois (Médecin Néphrologie Tours)
- Dr Abdelghani Fakhreddine Boustia (Médecin Radio-diagnostic Tours)
- Dr Kamel Walha (Médecin Radio-diagnostic Tours)
- Dr Fabrice Guerif (Pharmacien Tours)
- Dr Mohamed Hamza (Médecin Anesthesie-réanimation Tours)
- Dr Bruno Aesch (Médecin Neuro-chirurgie Tours)
- Julien Brans (Masseur-Kinésithérapeute Tours)
- Dr Nathalie Faure (Médecin Pédiatrie Tours)
- Dr ClÉmence Guillaume (Pharmacien Tours)
- Dr Stephanie Jobard (Médecin Médecine interne Tours)
- Dr Agnes Caille (Médecin Santé publique et médecine sociale Tours)
- Dr Laura Foucault-fruchard (Pharmacien Tours)
- Dr Yves Gruel (Médecin Qualifié en Médecine Générale Tours)
- Dr Arnaud Ciree (Pharmacien Tours)
- Cindy Saulnier (Sage-Femme Tours)
- Dr Elodie Bailly (Médecin Néphrologie Tours)
- Dr Sophie Watt-mairesse (Pharmacien Tours)
- Dr Caroline Di Guisto (Médecin Gynécologie-obstétrique Tours)
- Rosalie Tochet (Sage-Femme Tours)
- Katarzyna Talpain (Masseur-Kinésithérapeute Tours)
- Dr Corinne Rogez (Médecin Anatomie et cytologie pathologiques Tours)
- Dr Stephanie Benardeau (Médecin Anesthesie-réanimation Tours)
- Dr Samuel Majzoub (Médecin Ophtalmologie Tours)
- Julie Egea (Sage-Femme Tours)
- Dr Fares Safar (Médecin Anesthesie-réanimation Tours)
- Audrey Leday (Sage-Femme Tours)
- Dr Emmanuel Lescanne (Médecin Oto-rhino-laryngologie Tours)
- Dr Jean Krebs (Médecin Qualifié en Médecine Générale Tours)
- Dr Robert Courtois (Médecin Psychiatrie Tours)
- Dr Julien Cirier (Médecin Gynécologie-obstétrique Tours)
- Dr Gaelle Baty (Pharmacien Tours)
- Dr Martine Delain (Médecin Hématologie Tours)
- Dr Luc Bertram (Médecin Anesthesie-réanimation Tours)
- Dr Jean-marie Gouin (Médecin Oto-rhino-laryngologie Tours)
- Dr Laura Zaragoza (Pharmacien Tours)
- Dr Theodora Bejan Angoulvant (Médecin Cardiologie et maladies vasculaires Tours)
- Dr Emmanuelle Rault (Pharmacien Tours)
- Dr Jean-francois Valentin (Médecin Néphrologie Tours)
- Dr Emmanuelle Houy-durand (Médecin Psychiatrie Tours)
- Aurelie Genet (Masseur-Kinésithérapeute SAINT CYR SUR LOIRE)
- Dr Beatrice Birmele (Médecin Néphrologie Tours)
- Marlene Lacroix (Sage-Femme Tours)
- Dr Julie Delvallee (Médecin Gynécologie-obstétrique Tours)
- Betty Viaud (Sage-Femme Tours)
- Serge Barbaut (Masseur-Kinésithérapeute)
- Dr Alicia Liotard (Chirurgien-Dentiste)
- Dr Annie Tavernier (Médecin Gynécologie médicale)
- Dr Christophe Delesalle (Médecin Spécialiste en Médecine Générale)
- Dr Jean-francois Dailloux (Médecin Médecine Générale)
- Dr Judit Nagy (Médecin Spécialiste en Médecine Générale)
- Dr Olivier Arnaud (Médecin Radio-diagnostic)
- Dr Guillaume Nicolas (Chirurgien-Dentiste)
- Dr Stephane Berruer (Médecin Spécialiste en Médecine Générale)
- Dr Veronique Mercier (Médecin Ophtalmologie)
- Dr Franck Bruyere (Médecin Chirurgie urologique)
- Dr Thomas Desmidt (Médecin Psychiatrie)
- Dr Isabelle Lemane (Médecin Qualifié en Médecine Générale)
- Dr Nawel Benguella-mokadem (Médecin Anesthesie-réanimation)
- Dr Guillaume Bacle (Médecin Chirurgie orthopédique et traumatologie)
- Lucie Fouquet (Sage-Femme)
- Dr Helene Delacroix Maillard (Médecin Qualifié en Médecine Générale)
- Dr Marie-sophie Blanchere-sanouiller (Médecin Spécialiste en Médecine Générale)
- Dr Marion Pequin (Pharmacien)
- Dr Pascal Toban (Médecin Cardiologie et maladies vasculaires)
- Dr Dominique Zanardo (Médecin Chirurgie orthopédique et traumatologie)
- Dr Roger Pillore (Médecin Médecine du travail)
- Rachel Morin (Psychologue)
- Justine Arnaud (Psychologue)
- Valentine Marrier D Unienville (Psychomotricien)
- Wilfried Tranchemer (Infirmier)
- Anne Sophie Pouvreau (Infirmier)
- Alexandra Musikas (Orthophoniste)
- Coline Seguier (Orthophoniste)
- Mailys Memin (Orthophoniste)
- Enzo Clerici (Orthophoniste)
- Melissa Guisneuf (Diététicien)
- Gwladys Laubigeau (Psychomotricien)
- Celine Lamballais (Psychologue)
- Sylvie Halluitte (Orthophoniste)
- Christelle Renaudin (Infirmier)
- Agnes Rolin (Orthophoniste)
- Isabelle Verdeil (Psychomotricien)
Je souhaite éditer les informations de cette page
Avant d'aller plus loin, confirmez-vous que vous êtes bien propriétaire des données mentionnées sur cette page ? Seul le professionnel de santé en personne peut demander une modification de ses données personnelles.
Pour un affichage optimal, l'utilisation d'un ordinateur pour la mise à jour de vos informations est recommandée.
Je ne suis pas Dr Thomas DESMIDT. Je certifie que je suis Dr Thomas DESMIDT.
Souhaitez-vous modifier les informations présentes sur cette page (adresses d'exercice, numéro de téléphone, ...) ou supprimer définitivement votre page de mablouseblanche.fr ?
Je veux modifier ma page Je veux supprimer ma page
Modifier mes informations :
Les informations sont correctes Mettre à jour cette adresse


Nous vous informerons une fois disponible.
Doctoome > Psychiatre > Tours (37000) > Dr Thomas DESMIDT
Dr Thomas DESMIDT
+ 1.2 million de membres
La mission de Doctoome ?
Doctoome met gratuitement à votre disposition ses services pour vous trouver l'offre de soin personnalisée et disponible dans votre région.
- Trouver les praticiens adaptés à ma pathologie ou aux besoins de mon entourage
- Trouver et réserver mes médicaments disponibles tout de suite dans les pharmacies autour de chez moi
- Prendre soin de moi en m'accompagnant au quotidien dans mon suivi personnalisé
Toutes les informations du Dr Thomas DESMIDT
Adresse du cabinet médical.
2 Boulevard Tonnellé, 37000 Tours
Prise en charge
Horaires de consultation, présentation du docteur thomas desmidt.
Le docteur Thomas DESMIDT qui exerce la profession de Psychiatre, pratique dans son cabinet situé au 2 Boulevard Tonnellé à Tours. Le docteur ne prend pas en charge la carte vitale
Le psychiatre est le professionnel qui suivra votre santé mentale et qui sera à votre écoute. Après examen, il pourra poser un diagnostic de votre santé mentale, traiter et prévenir les troubles psychiques.
Prenez un rendez-vous en ligne dès à présent avec le Dr Thomas DESMIDT.
FAQ du Docteur Thomas DESMIDT
Derniers articles liés au métier de psychiatre.

Découvrez comment améliorer votre santé mentale avec des stratégies efficaces et des ressources utiles. Apprenez-en plus sur la dépression, l’anxiété et les troubles bipolaires, ainsi que sur les liens entre bien-être émotionnel et soutien psychologique. Consultez notre FAQ pour répondre à vos questions les plus courantes.

À l’occasion de Dry January, Marina Torre-Liabot revient sur les bienfaits de ce défi (auquel plus de 30% des français semblent s’intéresser en 2024), sur les différents types de consommation, à quoi il faut être attentif et comment se faire accompagner dans sa consommation, que l’on soit dépendant ou non.

Lorsque l’on est confronté à une crise d’angoisse ou d’anxiété, il est essentiel de savoir comment réagir de manière efficace et apaisante.
Ces autres psychiatres pourraient vous intéresser

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Original Article
- Published: 06 June 2017
Brain Tissue Pulsatility is Increased in Midlife Depression: a Comparative Study Using Ultrasound Tissue Pulsatility Imaging
- Thomas Desmidt 1 , 2 ,
- Bruno Brizard ORCID: orcid.org/0000-0002-0110-822X 2 ,
- Paul-Armand Dujardin 3 ,
- Redouane Ternifi 2 ,
- Jean-Pierre Réméniéras 2 , 3 ,
- Frédéric Patat 2 , 3 ,
- Frédéric Andersson 2 ,
- Jean-Philippe Cottier 2 , 4 ,
- Emilie Vierron 2 , 3 ,
- Valérie Gissot 3 ,
- Kang Kim 5 ,
- Howard Aizenstein 6 ,
- Wissam El-Hage 1 , 2 , 3 &
- Vincent Camus 1 , 2
Neuropsychopharmacology volume 42 , pages 2575–2582 ( 2017 ) Cite this article
1824 Accesses
25 Citations
32 Altmetric
Metrics details
- Brain imaging
- Cerebrovascular disorders
- Predictive markers
Cerebrovascular disease (CVD) is consistently associated with late-life depression but poorly documented in midlife depression. It can be hypothesized that the relatively low sensitivity of conventional neuroimaging techniques does not allow the detection of subtle CVD in midlife depression. We used tissue pulsatility imaging (TPI), a novel ultrasound (US) neuroimaging technique that has demonstrated good sensitivity to detect changes in the pulsatility of small brain volumes, to identify early and subtle changes in brain vascular function in midlife depression. We compared the maximum and mean brain tissue pulsatility (MaxBTP and MeanBTP), as identified by TPI, between three groups of middle-aged females matched for age: patients with depression ( n =25), patients with remitted depression ( n =24) and community controls ( n =25). MRI arterial spin labeling, white matter hyperintensities (WMHs) and transcranial doppler (TCD) were used as control conventional markers for CVD. We found no difference in the MRI and TCD measures among the three groups. In contrast, depressive patients showed an increased BTP related to the mean global brain pulsatility (MeanBTP) and no change related to large vessels (MaxBTP) in comparison with the remitted and control groups. US neuroimaging is a highly accurate method to detect brain pulsatility changes related to cerebrovascular functioning, and TPI identified an increased BTP in midlife depressed patients, suggesting early and subtle vascular impairments in this population at risk for CVD such as stroke or WMHs. Because high pulsatility could represent prodromal cerebrovascular changes that damage the brain over time, this paper provides a potential target for blocking the progression of CVD.
Similar content being viewed by others
Discovery and replication of cerebral blood flow differences in major depressive disorder
Late-life depression accentuates cognitive weaknesses in older adults with small vessel disease

Changes in cerebral connectivity and brain tissue pulsations with the antidepressant response to an equimolar mixture of oxygen and nitrous oxide: an MRI and ultrasound study
Introduction.
Cerebrovascular diseases (CVDs) are increasingly recognized as a part of the pathophysiology of late-life depression (LLD). In particular, the vascular depression hypothesis proposed by Alexopoulos et al (1997 ) has emphasized the role of CVDs in the predisposition, precipitation, or perpetuation of some geriatric depressive syndromes, and recent data suggest that vascular depression is a major health issue that may account for up to half of LLD ( Park et al, 2015 ). Subsequently, Krishnan et al (1997 ) have proposed a complementary definition of vascular depression based on neuroimaging and have suggested to use MRI as the reference for the characterization of CVDs in LLD. Since then, multiple studies have found a consistent association between LLD and MRI markers of CVDs, especially white matter hyperintensities (WMHs) ( Herrmann et al, 2008 ) ( Taylor et al, 2013 ).
In contrast, CVDs are poorly documented in early and midlife depression. However, growing evidence suggests that midlife-depressed patients constitute a group at risk for CVDs. Meta-analyses of epidemiological studies indicate that depression is a risk factor for stroke ( Pan et al, 2011 ) ( Dong et al, 2012 ). Preclinical and clinical studies suggest that depression is associated with pathophysiological mechanisms that may modify vascular function, such as inflammation ( Dantzer et al, 2008 ) or endothelial dysfunction ( Isingrini et al, 2009 ). Taylor et al (2013 ) recently reviewed the probable mechanisms linking vascular disease with depression and proposed that some underlying vascular processes, involving hypoperfusion, endothelial dysfunction and inflammation, progressively damage the brain and eventually lead to the development of WMHs and depression. However, this suggestion requires more clinical evidence, especially regarding the characterization of the underlying mechanisms linking depression with CVDs. Moreover, and to the best of our knowledge, no study has found signs of CVDs in midlife depression using neuroimaging.
There may be two reasons for the lack of evidence of CVDs in midlife depression: Either there is no CVDs or only late CVDs in early and midlife depression, or current neuroimaging techniques are not sensitive enough to detect early cerebrovascular impairments. Because WMHs are believed to represent late markers of earlier, progressive and insidious CVDs ( Wardlaw et al, 2013 ), the second assumption may prove to be true. Indeed, based on neuropathological data as well as data showing that the presence of cerebrovascular risk factor in midlife increases the risk of WMHs in late life ( Debette et al, 2011 ), a growing number of authors argue that WMHs are only the tip of the iceberg in terms of damage to the brain ( Wardlaw et al, 2013 , 2015 ; Hommet et al, 2011 ); the pathophysiology could begin years beforehand, with subtle changes in the vasculature that are invisible to conventional imaging and progressively lead to diffuse CVDs ( Wardlaw et al, 2013 , 2015; Hommet et al, 2011 ). However, conventional neuroimaging techniques may misidentify brain CVDs in early and midlife depression, possibly because their limited spatiotemporal resolution does not allow the identification of subtle and early stages of CVDs. Some recent ultrasound (US) neuroimaging techniques may prove to overcome this limitation and be sensitive enough to assess subtle cerebrovascular changes in early and midlife depression.
Indeed, the recent advances in US imaging technology, in both probe technology and signal processing, have made possible the development of so-called tissue pulsatility imaging (TPI). In principle, TPI is similar to transcranial doppler (TCD), except that rather than focusing on only large arteries, it relies on the Echo-B mode of a modern US scanner to measure the pulsatile movements of large brain regions, which allow a very high spatiotemporal level of detection (micrometers/millisecond) of brain volume changes related to pulsatile cerebral blood flow (CBF). Periodic changes in blood volume cause the brain to expand and relax over the cardiac cycle, and TPI measures the pulsatile signals from thousands of sample volumes in an US image plane, consistently with the principle of plethysmography ( Kucewicz et al, 2004 ). TPI has been validated on phantoms ( Kucewicz et al, 2004 ) and on healthy volunteers. It was shown that BTP was increased during visual stimulation ( Kucewicz et al, 2007 ) and was decreased during hyperventilation ( Kucewicz et al, 2008 ), which suggests that BTP is closely related to CBF changes and cerebrovascular reactivity (CVR), consistently with the notion that pulsatile CBF and cerebrovascular functioning are major determinants of brain pulsatility ( Wagshul et al, 2011 ). Our team also found that TPI was informative in clinical settings, as BTP was shown to be inversely correlated with WMH load ( Ternifi et al, 2014 ). In another pilot study ( Desmidt et al, 2011 ), we investigated the use of TPI in LLD, and we found that BTP was markedly lower in diabetic depressed patients compared with diabetic non-depressed subjects. Thus, TPI has been shown to identify changes in the BTP of patients with constituted CVDs, but to date, we lack data on the use of TPI on patients at risk of CVDs—including early and midlife depressed patients—who may exhibit early and subtle cerebrovascular changes.
The goal of our study was to characterize the BTP of midlife depressed females with TPI. Based on previous results on LLD and CVDs, we hypothesized that BTP would be lower in depressed patients compared with controls and remitted subjects. We also compared BTP with WMHs and CBF in the middle cerebral artery (MCA), as assessed by TCD and MRI arterial spin labeling (ASL), as potential cofounders of BTP ( Ternifi et al, 2014 ).
Materials and methods
Participants.
Seventy-five females aged 18–55 years were recruited and divided into three equal groups as part of the EMPHILINE project (NCT02026622 on clinicaltrials.gov), the principal objective of which was to investigate the cardiovascular and CVR in depression during emotional tasks; the baseline data are reported in the present article. To reduce variability, only females were recruited because previous findings have found significant differences in cerebrovascular properties between age-matched males and females ( Parkes et al, 2004 ). Subjects in the depressive group (group D; n =25) were inpatients or outpatients from the psychiatric inpatient units of the University Hospital of Tours, France. A psychiatrist (T.D.) diagnosed the patients as depressed according to the DSM-IV criteria for major depressive disorder (MDD) and a MADRS score higher than 21. The medical records of the psychiatric inpatient units were used to screen subjects for the remission group (group R; n =25). Subjects in the R group had to have at least one documented history of MMD in the past ten years but no criteria for current MDD in the last 6 months, and they needed a MADRS score lower than 9. Subjects in the control group (group C; n =25) were recruited from the local community and from the records of the research center of the Hospital of Tours, France. They had to have no history of MDD or any psychiatric disorder and have a MADRS score lower than 9. The three groups were matched for age. Non-inclusion criteria for all subjects were: (1) any history of psychotic, bipolar, or substance-abuse disorders or suspicion of severe cognitive impairment (MMSE <25), (2) any history of severe CVDs (myocardial infarction, arrhythmia, etc.) or neurological disorders (stroke, brain tumor, severe concussion, migraine, etc.), (3) any current instable medical condition, (4) current use of beta blockers or antipsychotics, (5) smoking over 10 pack-years, (6) auditory or visual impairments, (7) pregnancy or no reliable contraception, (8) contraindication for MRI, and (9) legal guardianship. The exclusion criterion was having no temporal window because US are attenuated by the thickness of the skull. Informed consent was obtained from all subjects, and the study protocol was approved by the local human ethical committee.
Clinical Assessments
Either a psychiatrist (T.D.) or a trained medical doctor (V.G.) from the research center performed the clinical and psychometric assessments in the research center of the University Hospital of Tours, France. Medical history and medication intake were recorded. The clinical assessment included blood pressure (measured twice, at rest and immediately before the US assessment), height and weight measurements. Psychometric assessments included the Structured Clinical Interview for DSM-IV (SCID) for current and lifetime MDD, the MADRS for severity of depression and the Mini Mental Status Examination (MMSE) for subjects over 50 to assess global cognitive functioning and screening for dementia.
Magnetic Resonance Imaging Protocol
MRIs were performed immediately after the psychometric assessment (except for five subjects for whom an MRI session was performed 1 week later owing to MRI unavailability) on a 3-Tesla Siemens Verio scanner (Siemens AG, Erlangen, Germany). T2-FLAIR sequence (fluid-attenuated inversion recuperation), ASL, and high-resolution T1-weighted MRI 3D volumes sequence (192 contiguous sagittal slices; 1 mm slice thickness; RT=1.9 s; TE=2.42 ms; TI=0.9 ms; FA=9°; in-plane resolution: 1 × 1 mm) were acquired for each subject.
WMHs Assessment
The WMH analysis method was based on the one described by Gurol et al (2006 ), using a FLAIR sequence (70 contiguous axial slices; 5 mm slice thickness; RT=9 s; TE=95 ms; TI=2.5 s; in-plane resolution: 1 × 1 mm). This method includes a visual and volumetric analysis, based on the fluid-attenuated inversion recuperation sequence. Three ROIs were located manually in a healthy WM ROI for each subject to obtain the mean intensity (mWH) and standard deviation (sdWM) of the T2-FLAIR value of the white matter tissue. Then, on each section, large and coarse ROI were drawn in areas that may contain lesions. Thresholding was then performed in the latter large ROI, using mWH+(3*sdWM) as a threshold value. A visual analysis of each section allowed the elimination of the gaps’ volume. The WMH volume was normalized by the total intracranial volume to correct for variations in head size.
ASL-MRI Assessment
Cerebral blood flow maps (relCBF) were computed from pASL sequences (45 pairs of label/control ASL images; 17 contiguous axial slices; 5 mm slice thickness; RT=2.6282 s; TE=13 ms; FA=90°; TI1=0.7 s; TI2=1.8 s; in-plane resolution: 4 × 4 mm; enabled prospective motion correction). For each subject, the CBF mean value was extracted from an ROI corresponding to MCA territory. Each individual MCA ROI was computed using the 3D-T1 image and based on Duvernoy’s work ( Anon, 2009 ) and Destrieux’s atlas ( Destrieux et al, 2010 ). A transformation matrix corresponding to the registration of ASL images to the structural 3D-T1 image was used to register each individual MCA ROI to each relCBF map.
US Protocol
US protocol was performed immediately after MRI sessions (except for five subjects who had their MRI session rescheduled; they had a US immediately after the clinical assessment) on an Antares medical scanner (Siemens Healthcare, Germany) by a single biophysics technician (B.B.), trained for TCD, who was blinded to the MRI results and the depressive status. Transcranial acquisitions were performed with a PX4-1 phased-array transducer (Siemens Healthcare; 1.82 MHz emission frequency, 70° field of view, 112 × 3 elements (1.5 D)). Measurements were performed through the right temporal bone window, which is the thinnest zone of the skull, with the probe positioned perpendicularly to the skull and maintained by a mechanical holder to reduce artifacts caused by the operator’s and the subject’s movements. Subjects were asked to remain in the supine position, try not to move, close their eyes and breathe normally.
The US scanner was first switched on Doppler mode for TCD acquisition. Color doppler was used to center the US beam on the right MCA. The pulsatility index (PI—ratio of the difference between maximal and minimal velocities over mean velocity) and the maximum and mean velocities of the right MCA were acquired and automatically computed by the scanner from a 10 s doppler sequence.
The US scanner was then switched to Echo-B mode to perform TPI measurements centered on the MCA. To limit US attenuation, we adjusted the depth of acquisition between 3 and 9 cm. With this configuration, we explored the circle of Willis and a transversal slice of the temporal hemispheres.
For each subject, the protocol consisted of four acquisitions of 10 s repeated every 26 s with an acquisition frame rate of 30 images/s (total of 297 frames). The US scanner provided direct access to beam-formed radiofrequency (RF) lines, which were used to estimate BTP. The data were then downloaded for offline analysis with MATLAB software (MathWorks, USA).
Our method described in the following lines assesses the axial component of brain displacements. Because the US beam is directed toward the center of the brain, BTP measures primarily relate to volume variations. Axial BTP was estimated using a 1D-intercorrelation method between successive acquisition frames. The maximum of the normalized intercorrelation coefficient, Txy/sqrt(TxxTyy), was used to estimate the delay between successive kernels on RF lines at a specific depth. The normalized correlation coefficient had to be higher than 0.7 to validate the measurement. A kernel size of eight periods (176 points, 4 wavelengths, 3.3 mm at 1.82 MHz) was chosen. Parabolic interpolation was performed around the three maximum points of the 1D correlation to improve the precision of the displacement measurement. With the signal to noise ratio obtained after propagation through the skull, the noise level of BTP was 0.8 μm (computed based on the Cramer-Rao lower bound formula, previously described for US measure of displacement in ( Byram et al, 2013 )). For a better spatial discretization along RF lines, an overlap rate of 80% of the kernel was computed, resulting in a 660 μm spatial discretization. A bandpass filter (low cutoff=0.75 Hz; high cutoff=5.0 Hz) was then applied to focus on displacements related to heart rate and to filter out slow and fast movements due to, respectively, respiration and artifacts.
We obtained a 3D matrix of BTP: axial displacements along the z axis (RF lines), x axis (along the 112 elements of the probe) and time. The temporal evolution of BTP was analyzed at each position of the 2D plane (curves of the displacement as a function of time). We used two criteria to filter out artifacts and focus on physiological signals, supposed to be stable in terms of periodicity and amplitude. The first criterion was used to investigate the periodicity of the brain pulsations and consisted in the ratio of the second maximum peak over the central peak (SMP/CP) of the temporal autocorrelation function of each kernel. If the ratio SMP/CP was higher than 0.6, the record was validated and confronted to the second criterion. This threshold of 0.6 was chosen based on previous studies ( Desmidt et al, 2011 , Ternifi et al, 2014 , Biogeau et al, in press ) and the empirical observations of several TPI signals. The second criterion was used to investigate the difference in amplitudes between pulsations in each curve. The cumulated standard deviation (CSTD) of the pulsations was calculated to inform on the data dispersion and was normalized to the peak-to-peak amplitude (Umean) of the mean temporal curve. If the ratio CSTD/Umean was lower than 0.25, the record was validated, otherwise rejected. This threshold of 0.25 was chosen based on the same reasons of the first criterion.
Finally, the medians (interquartile range) of the criteria showed similar values among groups, with no significant difference (for criterion 1: M=0.75(0.19), M=0.78(0.13), 0.79(0.16), for group D, R, and C respectively, p =0.993; for criterion 2: M=0.21(0.17), M=0.19(0.10), M=0.24(0.20), for group D, R, and C respectively, p =0.572), and suggested a significant stability in periodicity and amplitude.
From this final matrix, we isolated two curves, MaxBTP and MeanBTP, corresponding, respectively, to the curve with the maximum of the mean peak-to-peak amplitude (averaged between cycles) and to the averaging of all curves in the matrix. MaxBTP and MeanBTP thus correspond to the pulsatility of, respectively, the largest artery (ie, MCA) and the mean pulsatility in the whole region of acquisition. Finally, we calculated two parameters for each curve—the average peak-to-peak amplitude (BTP_PtoP) and the root mean square (BTP_RMS)—which reflect the largest amplitude and the displacement power value, respectively.
Statistical Analyses
Shapiro–Wilk tests determined that the variables were not normally distributed. We therefore performed non-parametric analyses for group comparisons (Kruskal–Wallis with Dunn’s post-test).
Clinical Characteristics
One patient in group R was excluded for having no temporal window. As shown in Table 1 , there was no difference in age, cardiovascular risk factors or baseline blood pressure among the three groups. Antidepressant intake was significantly higher in groups D and R compared with group C ( p <10 −4 ), but there was no difference between groups D and R ( p =0.857). The MADRS score was significantly greater in group D compared with group R ( p <10 −4 ), in group D compared with group C p (<10 −4 ) and in group R compared with group C ( p =0.011).
MRI and US Measures
As shown in Table 2 , all subjects except 2 (one in group D and one in group C, who had, respectively, 15.8 and 19.4 cm 3 total WMHs) showed very low WMHs, and there was no difference in the total or right hemisphere WMHs among the three groups. Similarly, there was no difference in MCA ASL CBF or in the TCD measures among the three groups. In contrast, as shown in Figure 1 , TPI measures showed a higher MeanBTP for both Peak-to-Peak and RMS values in group D compared with the two other groups, whereas we found no difference in the MaxBTP values. Finally, Figure 2 and Supplementary Video 1 show BTP color maps of three representative subjects in each group and provide visual examples of higher global brain pulsatility in depressed patients.
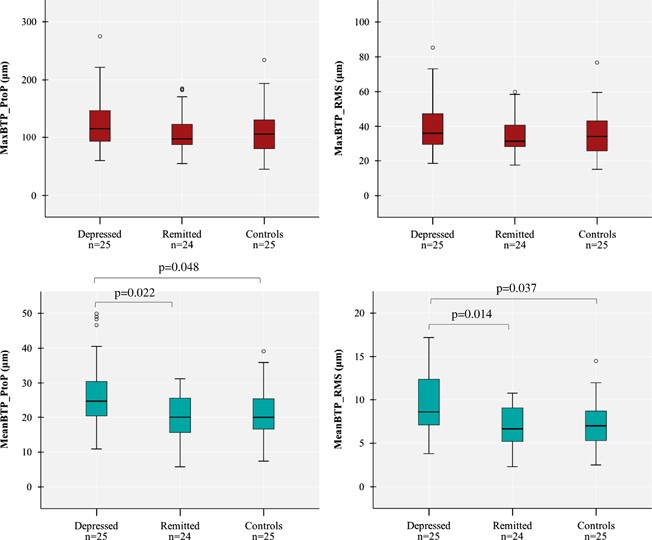
Comparisons of the TPI measures of MaxBTP (in red—top left: Peak-to-Peak, top right: RMS) and MeanBTP (in blue—bottom left: Peak-to-Peak, bottom right: RMS) among the three groups.
PowerPoint slide
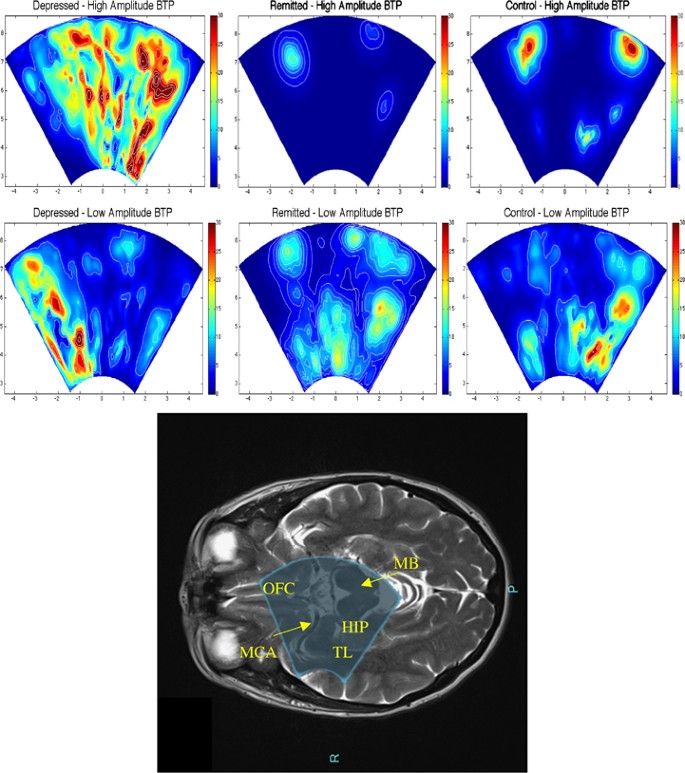
Brain tissue pulsatility (BTP) color maps from three representative subjects of each group and picture of the ultrasound beam of the TPI overlaid on a MRI axial image. We filtered BTP matrixes with a threshold equal to 50% of the MaxBTP of the depressed subject to distinguish between high- and low-amplitude BTP, shown, respectively, at the top and bottom of the figure. We also applied the Horn—Schunck method to estimate the optical flow and displacement speed of each volume in the region of acquisition, the color maps of which show maximum values. Depressed subjects (left) exhibit more distributed high BTP compared with remitted (center) and control (right) subjects, who, in contrast, exhibit very localized high BTP, probably around the main large arteries of the circle of Willis. Low BTP appears to be similarly distributed in each of the three subjects in various small regions, possibly corresponding to small vessels. These maps suggest that high brain pulsatility, normally limited to large vessels in remitted and control subjects, affects the whole brain in depressed patients, consistently with our result of a higher MeanBTP in depressed patients. As shown on the bottom figure, the ultrasound beam of the TPI overlays parts of the temporal lobes (including parts of the hippocampus), the orbitofrontal cortex and the brainstem (midbrain). Hip: hippocampus; MB: midbrain; MCA: middle cerebral artery; OFC: orbitofrontal cortex; TL: temporal lobe.
We found that MeanBTP was significantly higher in depressed subjects compared with controls and remitted subjects, whereas we found no difference in MCA CBF, as assessed by ASL, TCD, and TPI. MeanBTP corresponds to the averaged value of thousands of brain volume pulsations and informs on the mean global brain pulsatility related to both large and small arteries. In contrast, MaxBTP informs on only the largest pulsatility, which is likely due to MCA. Thus, our results suggest that midlife depression can be characterized by high global brain pulsatility without significant changes in large-artery CBF.
Growing evidence from the literature suggests that excessive intracranial pulsatility correlates with CVDs and brain atrophy. A recent study ( Wåhlin et al, 2014 ) that used MRI and invasive methods of pulsatility measurement on healthy volunteers found that higher intracranial cardiac-related pulsatility was associated with lower brain volume, especially in the hippocampus and temporal lobe, suggesting that excessive pulsatility correlates with atrophy. Similarly, other studies found that high pulsatility was observed in various CVDs, including WMHs ( Tsao et al, 2013 ) and cognitive disorders ( Sabayan et al, 2012 ) ( Tsao et al, 2013 ) ( Isingrini et al, 2009 ). However, these studies used TCD, which inform on only large-vessel pulsatility. In contrast, TPI provides direct monitoring of global brain pulsatility related to both large and small vessels, and, to our knowledge, our study is the first to show direct evidence of increased global pulsatility in midlife depressed patients, a group at risk for CVDs.
The observation that increased pulsatility is state dependent is consistent with findings that WMH progression is observed in individuals who remain depressed and do not respond to antidepressants ( Khalaf et al, 2015 ), suggesting that the longer one stays depressed, the worse their cerebrovascular health. Because high pulsatility could represent prodromal cerebrovascular changes that damage the brain over time and potentiate or mediate the progression of CVD such as WMHs, this paper provides a potential target for blocking the progression of CVD.
The result of an increased BTP in midlife depression is in contradiction with our a priori hypothesis. Nevertheless, this is the first study to investigate BTP with TPI in midlife depression and while there is a vast literature on CVDs in LLD, little is known about the cerebrovascular functioning in midlife depression. In a previous pilot study ( Desmidt et al, 2011 ), we found that BTP was decreased in LLD patients with long-term diabetes. In this pilot study, we did not control for WMHs and elderly diabetic patients are likely to exhibit high level of WMHs. As WMHs are strongly and inversely correlated with BTP ( Ternifi et al, 2014 ), the decreased BTP in LLD may indicate a larger WMHs volume in depressed patients, which is consistent with numerous studies that found a constant greater volume of WMHs in elderly depressed subjects compared with controls ( Taylor et al, 2013 ). In contrast, WMHs are rarely observed in midlife depression. WMHs consist in significant and irreversible structural changes that dramatically reduce BTP. However, recent findings suggest a pre-stage to WMHs that involves functional rather than structural changes, including excessive brain pulsatility ( O’Rourke and Hashimoto, 2007 ).
Taken together, our results suggest that there may be two profiles of BTP in depression, depending on the patient’s age and the presence of CVDs: low BTP in LLD patients with WMHs and high BTP in midlife depressed patients without WMHs. A study comparing BTP in midlife vs LLD, with WMHs as covariate, is required to confirm this suggestion. In addition, other studies involving depressed males would be required to investigate a possible gender effect of BTP on depression, as we recruited only females in our present study. Finally, longitudinal studies investigating the link between high BTP and the development of WMHs are required.
TPI has some limitations, including the inability to measure the whole brain in one acquisition, the inability to locate brain structures and limitations due to bone attenuation. However, it is an easy-to-realize, costless and non-invasive technique that could ultimately provide a sophisticated office tool for clinicians to detect subtle impairments in the cerebrovascular functioning of patients at risk for CVDs, especially depressive patients, to guide treatment and prevention strategies. We also foresee multiple perspectives for the development of TPI, including its coupling with MRI neuronavigation to allow anatomical localization, its use in preclinical settings to investigate molecular physiology that influences BTP, its implementation in large and longitudinal studies to assess BTP changes with treatment and symptoms remission in depression, and the development of sophisticated signal processing, such as frequency domain analyses.
Funding and disclosure
The authors declare no conflict of interest.
Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M (1997). “Vascular depression” hypothesis. Arch Gen Psychiatry 54 : 915–922.
Article CAS PubMed Google Scholar
Anon (2009) Internal Architecture of the Brain Stem with Key Axial Section. In: Duvernoy’s Atlas of the Human Brain Stem and Cerebellum . Springer: Vienna. pp 53–93.
Biogeau J, Desmidt T, Dujardin P-A, Ternifi R, Eudo C, Vierron E et al (In Press). Ultrasound tissue pulsatility imaging suggests impairment in global brain pulsatility and small vessels in elderly patients with orthostatic hypotension. J Stroke Cerebrovasc Dis 26 : 246–251.
Article PubMed Google Scholar
Byram B, Trahey GE, Palmeri M (2013). Bayesian speckle tracking. Part II: biased ultrasound displacement estimation. IEEE Trans Ultrason Ferroelectr Freq Control 60 : 144–157.
Article PubMed PubMed Central Google Scholar
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9 : 46–56.
CAS PubMed PubMed Central Google Scholar
Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C et al (2011). Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77 : 461–468.
Article CAS PubMed PubMed Central Google Scholar
Desmidt T, Hachemi ME, Remenieras J-P, Lecomte P, Ferreira-Maldent N, Patat F et al (2011). Ultrasound brain tissue pulsatility is decreased in middle aged and elderly type 2 diabetic patients with depression. Psychiatry Res 193 : 63–64.
Destrieux C, Fischl B, Dale A, Halgren E (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53 : 1–15.
Dong J-Y, Zhang Y-H, Tong J, Qin L-Q (2012). Depression and risk of stroke: a meta-analysis of prospective studies. Stroke J Cereb Circ 43 : 32–37.
Article Google Scholar
Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T et al (2006). Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 66 : 23–29.
Herrmann LL, Le Masurier M, Ebmeier KP (2008). White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry 79 : 619–624.
Hommet C, Mondon K, Constans T, Beaufils E, Desmidt T, Camus V et al (2011). Review of cerebral microangiopathy and Alzheimer’s disease: relation between white matter hyperintensities and microbleeds. Dement Geriatr Cogn Disord 32 : 367–378.
Isingrini E, Desmidt T, Belzung C, Camus V (2009). Endothelial dysfunction: a potential therapeutic target for geriatric depression and brain amyloid deposition in Alzheimer’s disease? Curr Opin Investig Drugs Lond Engl 2000 10 : 46–55.
CAS Google Scholar
Khalaf A, Edelman K, Tudorascu D, Andreescu C, Reynolds CF, Aizenstein H (2015). White matter hyperintensity accumulation during treatment of late-life depression. Neuropsychopharmacology 40 : 3027–3035.
Krishnan KRR, Hays JC, Blazer DG (1997). MRI-defined vascular depression. Am J Psychiatry 154 : 497–500.
Kucewicz JC, Dunmire B, Giardino ND, Leotta DF, Paun M, Dager SR et al (2008). Tissue pulsatility imaging of cerebral vasoreactivity during hyperventilation. Ultrasound Med Biol 34 : 1200–1208.
Kucewicz JC, Dunmire B, Leotta DF, Panagiotides H, Paun M, Beach KW (2007). Functional tissue pulsatility imaging of the brain during visual stimulation. Ultrasound Med Biol 33 : 681–690.
Kucewicz JC, Huang L, Beach KW (2004). Plethysmographic arterial waveform strain discrimination by Fisher’s method. Ultrasound Med Biol 30 : 773–782.
O’Rourke MF, Hashimoto J (2007). Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50 : 1–13.
Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB (2011). Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA 306 : 1241–1249.
Parkes LM, Rashid W, Chard DT, Tofts PS (2004). Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med 51 : 736–743.
Park JH, Lee SB, Lee JJ, Yoon JC, Han JW, Kim TH et al (2015). Epidemiology of MRI-defined vascular depression: a longitudinal, community-based study in Korean elders. J Affect Disord 180 : 200–206.
Sabayan B, Jansen S, Oleksik AM, van Osch MJP, van Buchem MA, van Vliet P et al (2012). Cerebrovascular hemodynamics in Alzheimer’s disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res Rev 11 : 271–277.
Taylor WD, Aizenstein HJ, Alexopoulos GS (2013). The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 18 : 963–974.
Ternifi R, Cazals X, Desmidt T, Andersson F, Camus V, Cottier J-P et al (2014). Ultrasound measurements of brain tissue pulsatility correlate with the volume of MRI white-matter hyperintensity. J Cereb Blood Flow Metab 34 : 942–944.
Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R et al (2013). Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81 : 984–991.
Wagshul ME, Eide PK, Madsen JR (2011). The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS 8 : 5.
Wåhlin A, Ambarki K, Birgander R, Malm J, Eklund A (2014). Intracranial pulsatility is associated with regional brain volume in elderly individuals. Neurobiol Aging 35 : 365–372.
Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc 4 : 001140.
Wardlaw J, Smith C, Dichgans M (2013). Mechanisms underlying sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 12 : 483–497.
Download references
Acknowledgements
This study received grant from the French National Research Agency (Agence Nationale de la Recherche: ANR-2011-EMCO-005-‘Emphiline project’) and the Planiol Foundation for brain study (Fondation Planiol pour le Cerveau), Varennes, France.
Author information
Authors and affiliations.
Clinique Psychiatrique Universitaire, CHRU de Tours, Tours, France
Thomas Desmidt, Wissam El-Hage & Vincent Camus
INSERM U930 Imagerie et Cerveau, Université François-Rabelais de Tours, Tours, France
Thomas Desmidt, Bruno Brizard, Redouane Ternifi, Jean-Pierre Réméniéras, Frédéric Patat, Frédéric Andersson, Jean-Philippe Cottier, Emilie Vierron, Wissam El-Hage & Vincent Camus
INSERM CIC 1415, Université François-Rabelais de Tours, Tours, France
Paul-Armand Dujardin, Jean-Pierre Réméniéras, Frédéric Patat, Emilie Vierron, Valérie Gissot & Wissam El-Hage
Service de Neuroradiologie, CHRU de Tours, Tours, France
Jean-Philippe Cottier
Department of Bioengineering, University of Pittsburgh School of Engineering, Pittsburgh, PA, USA
Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Howard Aizenstein
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Thomas Desmidt .
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Supplementary video (mp4 29629 kb), powerpoint slides, powerpoint slide for fig. 1, powerpoint slide for fig. 2, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Desmidt, T., Brizard, B., Dujardin, PA. et al. Brain Tissue Pulsatility is Increased in Midlife Depression: a Comparative Study Using Ultrasound Tissue Pulsatility Imaging. Neuropsychopharmacol. 42 , 2575–2582 (2017). https://doi.org/10.1038/npp.2017.113
Download citation
Received : 03 August 2016
Revised : 20 May 2017
Accepted : 30 May 2017
Published : 06 June 2017
Issue Date : December 2017
DOI : https://doi.org/10.1038/npp.2017.113
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Thomas Desmidt
- Paul-Armand Dujardin
- Helmet T. Karim
Molecular Psychiatry (2023)
Left amygdala volume and brain tissue pulsatility are associated with neuroticism: an MRI and ultrasound study
- Marta Andrea Siragusa
- Thomas Rufin
Brain Imaging and Behavior (2021)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Changes in cerebral connectivity and brain tissue pulsations with the antidepressant response to an equimolar mixture of oxygen and nitrous oxide: an MRI and ultrasound study
Affiliations.
- 1 UMR 1253, iBrain, Université de Tours, Inserm, Tours, France. [email protected].
- 2 CHU de Tours, Tours, France. [email protected].
- 3 CIC 1415, CHU de Tours, Inserm, Tours, France. [email protected].
- 4 CIC 1415, CHU de Tours, Inserm, Tours, France.
- 5 UMR 1253, iBrain, Université de Tours, Inserm, Tours, France.
- 6 CHU de Tours, Tours, France.
- 7 Behavior and Basal Ganglia Host Team 4712, University of Rennes 1, Rennes, France Department of Psychiatry, Rennes University Hospital, Guillaume Régnier Hospital Centre, Rennes, France.
- 8 Addictology and Liaison Psychiatry Department, CHU de Nantes, 44000, Nantes, France.
- 9 Department of Psychiatry, University Hospital, Angers, France.
- 10 Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
- 11 Department of Bioengineering, University of Pittsburgh, Pittsburgh, PA, USA.
- PMID: 37592013
- DOI: 10.1038/s41380-023-02217-6
Nitrous oxide (N 2 O) has recently emerged as a potential fast-acting antidepressant but the cerebral mechanisms involved in this effect remain speculative. We hypothesized that the antidepressant response to an Equimolar Mixture of Oxygen and Nitrous Oxide (EMONO) would be associated with changes in cerebral connectivity and brain tissue pulsations (BTP). Thirty participants (20 with a major depressive episode resistant to at least one antidepressant and 10 healthy controls-HC, aged 25-50, only females) were exposed to a 1-h single session of EMONO and followed for 1 week. We defined response as a reduction of at least 50% in the MADRS score 1 week after exposure. Cerebral connectivity of the Anterior Cingulate Cortex (ACC), using ROI-based resting state fMRI, and BTP, using ultrasound Tissue Pulsatility Imaging, were compared before and rapidly after exposure (as well as during exposure for BTP) among HC, non-responders and responders. We conducted analyses to compare group × time, group, and time effects. Nine (45%) depressed participants were considered responders and eleven (55%) non-responders. In responders, we observed a significant reduction in the connectivity of the subgenual ACC with the precuneus. Connectivity of the supracallosal ACC with the mid-cingulate also significantly decreased after exposure in HC and in non-responders. BTP significantly increased in the three groups between baseline and gas exposure, but the increase in BTP within the first 10 min was only significant in responders. We found that a single session of EMONO can rapidly modify the functional connectivity in the subgenual ACC-precuneus, nodes within the default mode network, in depressed participants responders to EMONO. In addition, larger increases in BTP, associated with a significant rise in cerebral blood flow, appear to promote the antidepressant response, possibly by facilitating optimal drug delivery to the brain. Our study identified potential cerebral mechanisms related to the antidepressant response of N 2 O, as well as potential markers for treatment response with this fast-acting antidepressant.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
- Antidepressive Agents / pharmacology
- Antidepressive Agents / therapeutic use
- Brain / diagnostic imaging
- Depressive Disorder, Major* / drug therapy
- Gyrus Cinguli / diagnostic imaging
- Magnetic Resonance Imaging / methods
- Nitrous Oxide* / therapeutic use
- Oxygen / therapeutic use
- Nitrous Oxide
- Antidepressive Agents
- Tour de France
- Stages - Results
- Previous winners
- Tennis Home
- Calendar - Results
- Australian Open
- Roland-Garros
- All Competitions
- Cycling Home
- Race calendar
- Vuelta a España
- Giro d'Italia
- Dare to Dream
- Football Home
- Fixtures - Results
- Premier League
- Champions League
- All leagues
- Snooker Home
- World Championship
- UK Championship
- Major events
- Olympics Home
- Mountain Bike Home
- UCI Track CL Home
- Men's standings
- Women's standings
- Alpine Skiing Home
- Athletics Home
- Diamond League
- World Championships
- World Athletics Indoor Championships
- Biathlon Home
- Cross-Country Skiing Home
- Cycling - Track
- Equestrian Home
- Figure Skating Home
- Formula E Home
- Calendar - results
- DP World Tour
- MotoGP Home
- Motorsports Home
- Speedway GP
- Clips and Highlights
- Rugby World Cup predictor
- Premiership
- Champions Cup
- Challenge Cup
- All Leagues
- Ski Jumping Home
- Speedway GP Home
- Superbikes Home
- The Ocean Race Home
- Triathlon Home
- Hours of Le Mans
- Winter Sports Home
- e-Sports Home
- Esport World Cup
Geraint Thomas outlines Tour de France plans for Ineos Grenadiers - 'I'll be there to help Carlos'
/dnl.eurosport.com/sd/img/placeholder/eurosport_logo_1x1.png)
Published 25/06/2024 at 20:07 GMT
Geraint Thomas has been outlining his expectations as he prepares for a 13th Tour de France. The Brit won the race back in 2018, before finishing second 12 months later. Now 38, expectations of topping the podium are low. But after being selected for Ineos Grenadiers, he says there is no reason why he cannot win a stage, while also revealing he'll "be there to help" team-mate Carlos Rodriguez.
‘It is my life’ - Cavendish relives his 34 Tour de France stage wins
'His body is saying no' - Sick Cavendish dropped as Tour swansong gets off to horror start
18 minutes ago
- Who is riding the 2024 Tour de France? Are Cavendish, Pogacar in the field?
- Kuss ruled out of Tour de France with Covid-19 in blow to Visma, Vingegaard
- Froome omitted from Israel-Premier Tech squad for Tour de France
Rider smashes teeth moments before Tour starts after fan knocks him off bike
34 minutes ago
Cavendish believes he will have 'five or six chances' to secure Tour stage win record
5 hours ago
'I went a bit too hot!' - Thomas reflects on fourth place in ITT

Boy George, Little River Band, Bonnie Tyler and Starship part of Timeless Summer Tour 2025
B oy George, Little River Band, Bonnie Tyler and Starship featuring Mickey Thomas will perform in New Zealand next summer as part of a new concert series celebrating legendary musicians from around the world.
The Timeless Summer Tour 2025 makes its debut in January packed with a stellar line-up of international superstars from the 70s and 80s who will perform in Christchurch on January 11, Napier on January 12, New Plymouth on January 16, Mount Maunganui on January 18 and Auckland on January 19.
Timeless Summer Tour promoter Glenn Meikle said he was excited to be bringing such a high calibre line-up to New Zealand.
“All of the artists performing at Timeless Summer Live are iconic musicians who have paved the way for many before them.
“Even though they were introduced to the world in the 70s and 80s, their music is just as impactful today as they were when they were released.
“Timeless Live is excited to have such an iconic line-up on our debut Timeless Summer Tour and we’re confident fans of all ages will enjoy the experience,” he said.
Tickets for Timeless Summer Tour 2025 go on sale from July 3, 2024.
Those who register for pre-sale access will have first dibs on tickets, with access to purchase tickets for two hours from 10am before they are released to the public.
Every ticket purchased in the first 48 hours will also come with a free Timeless Summer Tour fedora.
To register for the Timeless Summer Tour presale, go to www.timelesssummertour.com .
Boy George is a vibrant icon who has left an indelible mark on the pop and new wave genres. He rose to prominence as the lead vocalist of the band Culture Club, which he co-founded in 1981.
The band’s debut album, Kissing to Be Clever , catapulted them to international fame with hit singles like Do You Really Want to Hurt Me and Time (Clock of the Heart) .
Throughout the 80s, Culture Club dominated charts worldwide with subsequent albums such as Colour by Numbers and Waking Up with the House on Fire, delivering memorable hits like Karma Chameleon and The War Song .
Little River Band
Through the 70s and 80s, Little River Band (LRB) enjoyed huge chart success with multi-platinum albums and chart topping hits like Reminiscing, Cool Change, Lonesome Loser, The Night Owls, Take It Easy On Me, Help Is on Its Way, Happy Anniversary, We Two, Man On Your Mind, The Other Guy and It’s A Long Way There .
In 1982, LRB set a record for having had top 10 hits for six consecutive years. Bassist/vocalist Wayne Nelson joined the band in 1980, and worked alongside founding members with some of the most distinctive harmonies and vocal abilities, creating the unique LRB sound.
Bonnie Tyler
Bonnie Tyler is one of Wales’ best known performers, achieving chart success all over the world. She is recognised for her distinctive husky voice, and a long list of hit singles including Total Eclipse of the Heart , It’s a Heartache, Holding Out for a Hero, Lost in France, More Than a Lover, Bitterblue and If I Sing You a Love Song .
In her 50-year career, Tyler has performed for audiences in countries across the world, and she has enjoyed critical acclaim for her recent albums Rocks and Honey and Between the Earth and the Stars .
Starship featuring Mickey Thomas
Mickey Thomas is the owner of the soaring voice that propelled Starship through the 80s.
He made his mark in 1976 as lead vocalist on the mega-hit Fooled Around And Fell In Love with The Elvin Bishop Band before joining Jefferson Starship as the lead vocalist in 1979, recording a string of hits including J ane, No Way Out, Find Your Way Back, Stranger and Layin’ It on the Line .
The group was renamed Starship in 1985 and went on to record three #1 hit songs including We Built This City, Sara and Nothing’s Gonna Stop Us Now from the film Mannequin , which also was an Academy Award nominee.
Their hit It’s Not Over ‘Til It’s Over became Major League Baseball’s theme in 1987.
Timeless Summer Tour 2025 dates:
January 11 - Christchurch at QEII
January 12 - Napier at Church Road Winery
January 16 - New Plymouth at Bowl of Brooklands
January 18 - Mount Maunganui at Mercury Baypark
January 19 - Auckland at Auckland Showgrounds


- Standard et urgences
- --> --> --> Espace patient --> -->